
Mascara © brunobarillari / fotolia.com
Carbon Black or carbon soot is a material with high economic importance containing pure carbon. Carbon Black is produced through specific combustion processes and the production of this material can be traced back more than one hundred years. It is used in many products including car tyres, printer toner, dyes for leather or textiles and mascara.
How can I come into contact with this material?
As carbon black is used in many products, it is possible for it to be taken into the body through a range of exposure routes. These can include breathing (inhalation), for example from laser printer emissions, or through the skin (dermal uptake) when contact is made with cosmetics and textiles. It is worth noting that swallowing of carbon black (oral uptake) and uptake into the gut is not a common route of uptake for carbon black nanoparticles. Those at greatest risk from coming into contact with this material are the personnel who work in environments producing carbon black nanoparticles. This is a major topic in industry, as several million tons of these particles are produced per year and many studies have been carried out to measure the concentration of carbon black particles in the air and to investigate the possible impact these may have on the workers. When looking at the effects of carbon black on human health the environmental release of carbon particles, such as ultrafine dust from combustion processes or traffic seems to be a bigger issue than manufactured carbon black nanoparticles released from controlled production processes.
How dangerous is this material for humans and the environment?
Several points need to be taken into consideration when analysing the effects of carbon black in humans, animals or the environment. The purity level of the material is one consideration. Carbon black nanoparticles of high purity cause responses in organisms only at very high concentrations which are considered to be environmentally unrealistic. However, carbon black may contain contaminants either in the carbon material or on the surface of the particles themselves. Fine dust particles (from sources such as industry exhaust gases, car exhausts and cigarette smoking) consist of amorphous carbon and these particles may be loaded with other chemicals. These could enhance any potentially harmful effects on living organisms. Never the less it is possible that pure carbon black nanoparticles may have adverse lung effects when inhaled in large amounts.
Conclusion
Humans may have frequent contact with carbonaceous particles in the air but compared to particles from combustion processes and other unintended environmentally relevant release sources, pure carbon black has a less critical biological effect.
By the way…
Tattoo dyes may contain a high concentration of carbon black as it is often used as the black pigment.
Properties and Applications
Carbon Black (CB) is a specific type of elemental carbon in the form of colloidal particles that is generated or produced through incomplete combustion processes or the thermal decomposition of gaseous or liquid hydrocarbons under controlled conditions. It occurs as a black fine dusty powder that must be differentiated from the undefined byproducts soot and diesel exhaust particulates generated during coal or hydrocarbon combustion. Carbon black consists of more than 96 percent of amorphous carbon and of small quantities of oxygen, hydrogen, nitrogen, and sulfur. Most of these elements are concentrated on the surface. Subsequently oxidized carbon black may contain up to 15 percent of oxygen.
Carbon black can be tailored to the respective intended purposes, hence is characterized by a high diversity that is essentially determined by the method of production and the variation of the process parameters. Carbon black consists of chain-type or botryoidal aggregates that have coalesced during production from smallest, mostly spherical particles.While still in the reactor, these aggregates form large agglomerates. The types of carbon black that have a high specific surface and widely ramified aggregates are particularly conductive. These conductive carbon blacks are used, for example, in antistatic finishings of plastics. For many applications, the carbon blacks are subjected to aftertreatments. In paints and varnishes with high color intensities, one uses e.g. carbon blacks that have been enhanced through subsequent oxidation.
Often, the formation and sizes of the aggregates described above require thorough explanation. The end product of carbon black is frequently described to be less than 0,1 micrometers in aerodynamic diameter. As a matter of fact, carbon black is produced from small spherical particles (so-called primary particles or nodules) with sizes in the range of 15–300 nm. These particles melt into particle aggregates that are 85–500 nm in aerodynamic diameter. Due to strong electric forces, these aggregates remain closely bound, forming even large agglomerates with other aggregates. Since these latter processes occur during production and since the agglomerates, once formed, do not break apart anymore, all commercial carbon blacks consist of 1 – 100 µm sized agglomerates.
The imgae shows the different stages of carbon black structure development. To ensure easy handling and avoid the occurrence of dusts, carbon blacks have been distributed for some time as pellets sized 0,1 to 1 millimeters. The use of ultrafine primary particles is restricted to the production furnace.
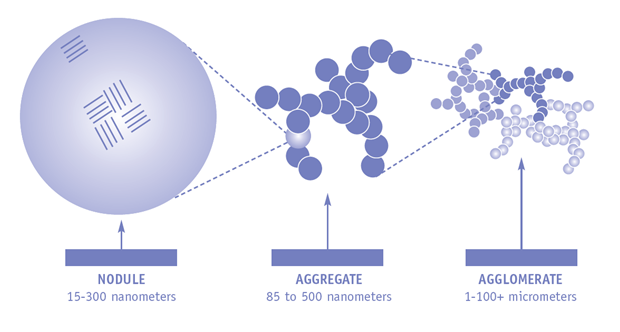
Sequence of structure development of Carbon Black. © International Carbon Black Association (ICBA). |
More than 90 percent of all carbon black is used as filler in the rubber industry, mainly in tires and technical rubber products such as conveyor belts, flexible tubes, and sealing profiles. Moreover, carbon black is used as black pigment in printing inks, India inks, paints and varnishes, for dyeing and for UV protection of plastics as well as in special products such as mascara, flower soil, decor paper, and fibers. As conductive carbon black, it is used in the electrical industry to manufacture electrodes and carbon brushes.
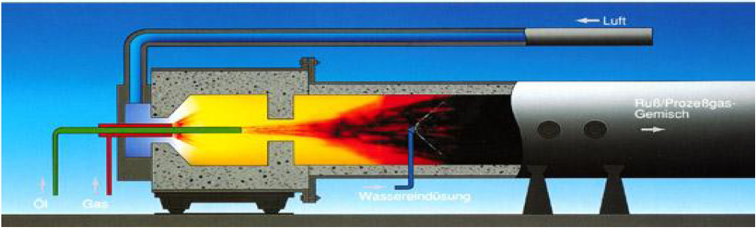
Scheme of a furnace black reactor. © Evonik Industries AG. |
Carbon black is not self-inflammable. Under the influence of an ignition source a mixture of air and graphene is explosive (dust explosion). The behaviour in a dust explosion is similar to that of other, carbon-based materials.
Production
Currently, carbon black is among the 50 most produced chemicals (8.1 million t/a) in the world. More than 90 percent are used in the rubber industry. In China, “lamp black”, the forerunner of today’s carbon black, was manufactured already more than 3,500 years ago [4].
Since the middle of the 1970s, the annual bulk of carbon black (approximately 98 percent) has been supplied by the furnace process where a hot gas with temperatures in the range of 1200 to 1800°C is generated in a furnace through combustion of natural gas or oil. Coal- and oil-based carbon black oils that are rich in aromatic compounds are injected in the produced hot gas. In addition to hydrogen and other gaseous compounds, carbon black is generated through the incomplete combustion and thermal fission (pyrolysis) of the feedstock. After an exactly defined reaction time, the reaction is stopped by abrupt cooling down (quenching) of the process gas mixture through injection of water. In baghouse filters, the product is then separated from the process gas. The furnace reactors are operated nonstop in shifts. Other carbon black production methods include gas or channel black, lamp black and thermal black procedures.
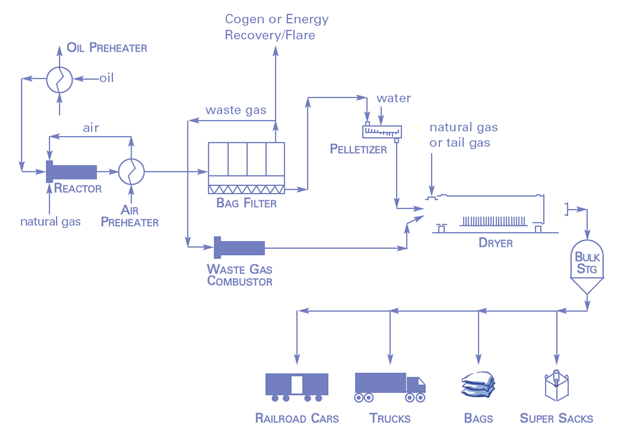
Typical furnace black process diagram. © International Carbon Black Association (ICBA). |
NanoCare Data Sheet
Further Information
- International Carbon Black Association (ICBA) (Juni 2004). Carbon Black User’s Guide - Safty, Health & Environmental Information.
Studies explicitly dealing with the release of nanoscale carbon black from products during use are currently not available. In small doses, carbon black does not cause any harm.
General Hazards
An epidemiological study on carbon black exposure in carbon black-producing factories in England showed that some workers after increased exposure were suffering from cough, sputum production, and a decreased lung function . In Canada and the USA, another study revealed a correlation between the occurrence of chronic obstructive pulmonary diseases and increased carbon black exposure . Besides, studies of printing ink proved the carcinogenic effect of carbon black in mists of ink . A case study published in 2010 states that carbon particles from toners that have been inhaled can affect the health of the persons exposed .
The International Agency for Research on Cancer (IARC) has classified carbon black used in toners into group 2B. This means that „carbon black is possibly carcinogenic to humans”. However, IARC at the same time says that „there is inadequate evidence in humans for the carcinogenicity of carbon black“.
Another study carried out in 2001 shows that there is no correlation between lung cancer and carbon black . It seems that mixtures of the type used in toners may be more critical than pure carbon black.
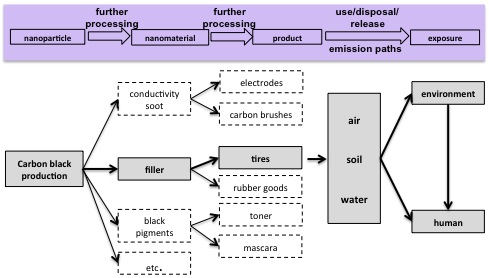
Life cycle and possible release paths of nanoscale carbon black from products shown here as an example for tires. © Kuhlbusch 2010, UBA Study . |
In 2004, Kuhlbusch et al. have shown that mainly carbon black-containing particles > 400 nm are released during carbon black bagging operations . Further studies by the authors reveal that during carbon black production, no release of nanoscale carbon black is expected to occur under undisturbed conditions. However, a release may occur as a result of leakages or during accidents .
Studies on Living Organisms – in vivo
In principle, Carbon Black can get into the organism via all entry portals such as the lung, the intestinal tract, and the skin (if it is injured). Most frequently, Carbon Black is taken up by the respiratory tract. It was found that high doses of inhaled Carbon Black do not cause damage to human organisms and the organisms of animals . Only very high doses can cause damage to the lung or lead to tumors of the lung in the extreme case.
In long-term studies where rats were made to inhale high doses of up to 1,1 mg/cm3 of Carbon Black for six hours/day on five days/week over 13 weeks with convalescence periods of three and eight weeks, no harmful effects were observed to occur. Only at still higher concentrations (> 2,5 mg/cm3), cell and tissue damage through to lung tumors were found to occur in the animals .
While in the case of inhalation, test animals are made to take in particles through the nose for a certain period of time, a particle suspension is instilled in the nasopharyngeal zone during instillation. Very high doses of nanoscaled Carbon Black administered by instillation cause an increase in the leukocytes which are known to be inflammation tracers . Extreme doses can cause tissue damage and, moreover, strongly impair the phagocytosis capability of the macrophages . Lungs of mice instilled with Carbon Black (0,1 - 0,5 mg/lung), however, did not show any signs of inflammation. Deposits of the particles showed as local black spots in the lung tissue .
Studies Outside the Organism - in vitro
Different studies conducted in the recent years have shown that certain doses of nanoscale carbon black (primary particle size approx. 14 nm), also referred to as ultra-fine carbon black in previous investigations, cause more oxidative stress and are more toxic to cells than coarser particles (> 100 nm) . Dose-dependent cytotoxicity, secretion of inflammatory markers, and reactive oxygen species were detected for different cell types . Pulskamp et al. have shown that in macrophages of the rat and in human epithelial lung cells, defined doses of 14 nm carbon nanoparticles can cause formation of dose-dependent reactive oxygen species (ROS) and that, in both cell lines, cell activity decreases accordingly . Other studies show that, depending on their type and origin, different cell types can react differently on the treatment with such particles .
Yet a further study observed an increase in apoptotic cells followed by apoptosis – the programmed death of cells - in cell layers treated with high concentrations of 20 µg of carbon black per cm2. During apoptosis, the damaged cells start shrinking, the DNA decomposes, and the cell destroys itself.
It has been reported by several authors that carbonic particles or fibers can cause problems in cell culture test systems . The false-positive, invalid results obtained due to interferences of the particles with pigments or dyes prevent one from making explicit statements on the toxicity of carbonic particles.
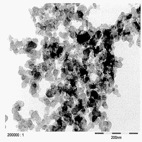
TEM image of Carbon Black agglomerates. © NanoCare Final Scientific Report
With this in view, the MTT assay was replaced by the WST test (water soluble tetrazolium) within the NanoCare project and at least one additional vitality test was carried out to obtain valid results.
Neither did any of the eleven cell lines of different origins tested in layers containing up to 10 µg of carbon black particles per cm2 exhibit stress symptoms nor did carbon black cause any cell-damaging effects. Moreover, a test for cell culture apoptosis proved negative. As shown in other studies, carbon black caused the formation of reactive oxygen species. Further in vitro experiments on human lung cells proved that the latter do not get stressed before being exposed to high doses of 25 µg particles per cm2 cell layer. The cell vitality was observed to decrease strongly at and above such particle concentrations .
Within NanoCare complex, so-called co-culture systems were used in addition to simple culture systems with only one cell line. By simulating the interaction of the cells, such systems allow a better representation of the in vivo situation in the body. The carbon black particles were found to trigger small biological effects in the respective systems .
In general, it could be shown that carbon black tends to agglomerate considerably. The agglomerates can be detected in the cells. Medium doses of carbon black cause the formation of ROS while high doses can damage cells in in vitro experiments . In addition, a genotoxic effect is described for high concentrations of carbon black contained in toners together with several other components (e.g. artificial resins, magnetizable metal oxides, dyes, and other additives) .
It is difficult to distinguish between an environmental exposure with synthetically produced Carbon Black (CB) and soot from combustion processes (e.g. traffic exhaust). The latter contains many harmful substances produced by combustion processes, which bind effectively to the carbon particles.
Soot from different sources is found in all environmental compartments, such as air, rain and surface waters, and in soil . To date, data on environmental concentrations of engineered Carbon Black nanoparticles are not available.
In principle, carbon black can be absorbed by breathing air, by ingestion or via the skin.
Uptake via the Lung – Inhalation
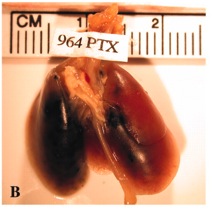
Lung of a mouse after instillation with carbon black. The left part of the lung shows massive black deposits; the right part exhibits only few deposits around the inlet/outlet of the major air passages and blood vessels (lung hilum).© Lam et al., 2004.
Carbon Black is taken up mainly via the respiratory air. Effects on health seem to increase with the age of the person inhaling the particles . Epidemiological studies have shown that high concentrations of carbon black can cause cough, sputum production, bronchitis, and even lung cancer . Also in vivo studies reveal that inhaled particles deposit in the lung and can be detected as black spots in the lung tissue . While small doses of inhaled carbon black can be found as deposits in the lung tissue without causing any damage, high doses may lead to inflammation, tissue injury or even lung tumors .
Uptake via the Skin - Dermal Uptake
As shown by the European project NanoDerm for titanium dioxide particles, the skin generally provides a very good barrier to nanoparticles . There has been no evidence so far of particles or nanoparticles transferred via the skin to blood vessels or close-to-surface cells of the immune system such as the dendritic cells (Langerhans cells). Skin cells exposed to concentrations of 10 µg particles per cm2 during in vitro studies did neither exhibit stress symptoms nor did the carbon black cause cell-damaging effects. Moreover, an in vitro test for apoptosis proved negative .
A conclusion by analogy puts it all into perspective: Tattooing ink, particularly black ink, contains carbon particles (carbon black consists of more than 96 % amorphous carbon) and is deposited in the deeper layers of the skin. The fact that only very few of these particles are transported from there into the nearby lymphatic vessels while most of them remain where they are explains the lifelong durability of the tattoos.
Uptake via the Gastro-Intestinal Tract
Studies of the uptake of carbon black in calves have shown that the particles are mainly taken up by the small intestines’ Peyer’s patches . Cell cultures of large-intestine cells exposed to concentrations of 10 µg particles per cm2 did neither exhibit stress symptoms nor did the carbon black cause cell-damaging effects. Moreover, a cell culture test for apoptosis proved negative .
For the larvae of the fruit fly, nanoscale Carbon Black (CB) administered in feed, was non-toxic, and the development and fertility of adult flies was not affected. However, particles enriched in the fly’s body and were still visible in adult animals as a distinct black coloration in the body.

The common mussel in water containing carbon black. The filter feeders try to get rid of the particles by excreting mucus (arrows). © Canesi et al., 2010.
The particle powder adhered quickly and firmly to the exterior of adult exposed animals, and removal by the natural grooming behavior was not possible. The particle-coating led to impaired mobility and killed the animals within hours by blocking the breathing holes . Such an exposure scenario to large amounts of pure Carbon Black is highly unlikely under real environmental conditions. Ground-dwelling amphipods also showed an increased mortality when exposed to very high concentrations of Carbon Black .
A brown algae, the toothed wrack was selected as a marine model organism, and effects on fertilization, embryo development and germination in the presence of nano-and microscale Carbon Black has been investigated . No uptake of nanoscale Carbon Black was observed, but very high concentrations prevented the fertilization and development. However, germination and root growth were unaffected.
Since mussels feed by filtering smallest particles from the water, they are considered particularly at risk by expsoure to engineered nanoparticles. Carbon Black strongly influenced the immune system and certain digestive processes of animals, indicating an uptake of the particles from the water .
Soot from exhaust gases, which was not precisely defined in terms of size, reduced the toxic effect of a weed control agent to a green algae . This effect can be explained with the strong binding of chemicals to soot particles, making them unavailable for the algae and therefore no longer toxic.
In conclusion, nano-scaled, pure Carbon Black as a material is little toxic for the organisms studied. Harmful effects can be found, however, due to the strong binding of the soot on surfaces and chemicals.
Nanoscale carbon black can be incorporated into cells.
Behaviour at the Blood-Brain Barrier
It was shown in animal experiments that different types of nanoparticles instilled in the trachea can get into the lung’s blood vessel system. Once in the blood, these nanoparticles can pass the blood-brain barrier to get into the brain tissue and trigger inflammatory reactions. Nanoparticles passing the blood-brain barrier have also been shown in a tissue model.
According to an in vivo study with mice, nanoscale carbon black particles instilled in the nose can induce inflammation markers in the olfactory nerve. The authors assume that the particles are taken up in the olfactory mucous membrane in the upper part of the nose by ends of the olfactory nerve and are transported into the brain where microglial cells, the macrophages of the brain, are activated and then release inflammation markers. No inflammation markers were detected, however, in other areas of the brain such as the hippocampus .
Behaviour of Uptake in somatic cells
Individual particles of carbon black or agglomerates of carbon black can be taken up in cells through different active or passive uptake processes. The nanoparticles mostly occur in vesicles or freely in the cytoplasm. The different uptake processes are described comprehensively in the literature . Considering the different cellular effects of the particles, these uptake processes are assumed to play a role beside the particle type and size.
Carbon black tends to agglomerate considerably. The agglomerates are taken up by the cells and can be detected in vesicles by means of electron microscopy. The vesicle membrane protects the remaining cell components from the particles, i.e. the latter are found in the cell in an encapsulated state . Low doses of carbon black stimulate the phagocytosis activity of the macrophages whereas very high doses considerably impede the phagocytosis capability of the macrophages .

Embryo of the toothed wrack with nanoscale carbon black agglomerates (left), A toothed wrack plant (right). © Nielsen et al., 2008.
Carbon Black (CB) has the ability to excellently bind many organic substances; therefore it is used in many industrial applications as a filter material (activated carbon). While the removal of pollutants in industrial processes is targeted, this process happens randomly in the environment.
A wide variety of pollutants (such as pesticides, drugs or exhaust gases) is capable to bind to the soot particles and influence their toxic effects . Soot particles having a primary size of 14 or 260 nm form very large agglomerates of 800-1300 nm size in saline seawater .
 >
>