Tire wear particles – an underestimated issue for humans and the environment?

The emission of tire and road wear particles (TRWP) is linked to car driving, especially under stress driving conditions. These particles pose risks to various ecosystems and environmental organisms, and their release and distribution patterns exhibit significant variability depending on driving behaviours, tire types, and road conditions. Utility items are subject to constant wear and […]
Read moreMaterials for 3 D printing

3D printing has boomed in recent years, even for private use, as 3D printers and printed materials are available at low cost. 3D printing here refers primarily to the low-cost FFF process. In this process, a three-dimensional part is created from a plastic that is available in a suitable form, e.g. as a wire on […]
Read moreAirborne Fibres

In newspapers or on the internet the headline regularly appears saying that nanomaterials behave like asbestos in organisms and induce a rare form of cancer called “Mesothelioma”. What is the story behind such news, and should we be concerned about this? Fibre dust and the asbestos problem To answer this question, we should first […]
Read moreGranular biopersistent dust particles

There are millions of workplaces with a substantial dust burden not only in Germany. As dust affects the lung and has negative effects on the health of workers, specific occupational exposure limits are defined, which are adapted permanently to actual research results. It is simply the dust particles, so-called GBP, and they must not contain […]
Read moreLabelling of Chemicals and Additives

The numbers described here, i.e. E Numbers, CI Numbers, and CAS Registry Numbers, identify additives in products on the market. In food technology, E numbers are applied to food packaging to indicate permitted food additives. Additives in cosmetics are alternatively labelled with INCI names (International Nomenclature of Cosmetic Ingredients) or CI numbers (Colour Index). The […]
Read moreNanoplastic in the environment

Plastic is ubiquitous as packaging material or as part of many products of our daily life. However, due to the steadily increasing global plastic production, plastic particles can now be found everywhere in the environment. It is estimated that between four and twelve million tonnes of plastic enter the seas and oceans every year [1]. […]
Read moreNanomaterials in the wastewater treatment plant

Engineered nanomaterials can be released from nanoproducts during use and waste disposal and a fraction can end up in wastewater. The function of wastewater treatment plants is to purify polluted water which includes the removal of nanomaterials. However, they can potentially negatively affect the purification potential of wastewater treatment plants How do nanomaterials get into […]
Read moreNanomaterials in plant protection products

Pesticides or plant protection products are intended to protect plants from pests such as insects, microorganisms or vermin such as nematodes. The use of nanomaterials in this sector is based on a number of expected benefits, such as an increase in pesticide efficacy against pests which allows for the application of lower amounts of pesticides. […]
Read moreNanomaterials and pollinating insects

Pollinating insects like bees or bumblebees are potentially exposed to nanomaterials via aerosols, the pollen of contaminated plants and water droplets. Managed pollinators may be additionally exposed in the hives due to direct application of nanomaterials by beekeepers. There is concern that pollinating insects are at risk due to nanomaterial exposure Decline of pollinating insects […]
Read moreTransformation of nanomaterials in the environment

After their release, innovative materials or nanomaterials are not only distributed in the air, soil or water (see Basic Information: How are innovative materials (e.g. nanomaterials) released?) but can also be changed, i.e. they undergo transformation processes (so-called transformation). These are processes in which the materials interact with the environment and are changed. Transformation can […]
Read moreEstimating the occurrence of nanomaterials in the environment

To perform a risk assessment of nanomaterials in the environment, information on the exposure, i.e. the amounts that are present in the environment, is essential. In contrast to many other known pollutants, the concentrations of nanomaterials in environmental systems cannot be measured directly. In this situation, exposure modelling is a solution to estimate the environmental […]
Read moreDetecting nanomaterials in the environment

Engineered nanomaterials are intentionally and unintentionally released into the environment, which might pose a risk to the ecosystem. Nanoparticulate iron for example is applied as remediation agent and intentionally released in the groundwater in order to remove harmful chemicals. On the other hand, nanomaterials are emitted unintentionally by disposal of nanomaterial-containing products or from wastewater […]
Read moreIn vitro tests for the benefit of nanosafety

In vitro tests are used in many fields of the biological sciences, since they are usually quick and relatively inexpensive. The term in vitro test designates the investigation of biological processes outside of an organism. These tests are extensively used in medical drug development. Besides of their merits, however, there are also limits for their […]
Read moreNanomaterials in food packaging

Packaging is essential for the distribution and storage of food. Its main function is to maintain the quality of food in terms of freshness and the absence of pathogens. Packaging material are made of paper, glass, metal or plastic. Often different packaging materials are combined to provide better protection. Its purpose ranges from protective abilities […]
Read moreNanomaterials in Food

Certain nanomaterials can be used in food and food products for various purposes. In the form of approved food additives, they alter properties such as taste, appearance or shelf life of the food. They are also used to encapsulate essential trace substances, making them more stable and easier for humans to absorb. At present, however, […]
Read moreNanomaterials in waste

New products containing nanomaterials are constantly being released to the market and used for diverse purposes. As a result, growing amounts of products containing nanomaterials enter the end-of-life phase of the lifecycle and are disposed. Nanowaste in solid or liquid form is currently treated as normal waste and is hence disposed via the existing waste […]
Read moreToner

Toners are very fine powders mainly used in copy machines and laser printers. They are often composed of particles with 2-30 microns in diameter. The small particle sizes cause the powders to behave like liquids. Consumers can come into contact with toner particles or paper dust during maintenance work in the resting state of the […]
Read moreNanoparticles in Textiles

The textile industry is one of the most important industries for consumer goods worldwide generating textiles for clothing, household goods, furnishing and technical purposes. Like other chemical processes and technologies, nanomaterials are used to add or improve different functionalities of the textiles. These materials could have an adverse effect on humans and environment. Proper selection […]
Read moreNanoparticles in paints

In general terms paint depicts a liquid that is used to coat a solid surface in order to protect, seal or colour it. For this purpose pigments such as solid particulates play an important role and have been used since millennia e.g. for cave painting. Today, nanomaterials are being used in order to improve the efficiency and […]
Read moreRisk Analysis of Nanomaterials

Risk analysis is broadly defined to include risk assessment , risk characterization, risk communication, risk management, and policy relating to risk, in the context of risks of concern to individuals, to public- and private-sector organizations, and to society at a local, regional, national, or global level [1]. In general, the term “risk” denotes the potential […]
Read moreRisk Assessment of Nanomaterials

Risk is defined differently depending on the (scientific) discipline. In general, the term “risk” denotes the potential loss of something of value, e.g. health or an intact environment or simply your purse. A risk exists only if hazard and exposure occur together. In toxicology, the term risk describes the function of the probability of “exposure” […]
Read moreRisk Management of Nanomaterials

The definition of a level of acceptable risk of new nanomaterials is defined on a societal consensus and political decision based on scientific risk assessment. The measures to reduce or prevent risks are part of adapted or new laws and regulations which stand at the end of a sophisticated process called risk management. During the […]
Read moreIronoxide carbohydrate nanoparticles for iron deficiency
The application of metallic nanoparticles is being widely investigated for various nanomedicines. They offer the possibility to develop both novel diagnostic and therapeutic tools. One of the first products on the market were iron-based nanoparticles for treatment of iron deficiency. Intravenous iron-carbohydrate nanoparticles for the treatment of iron deficiency Iron deficiency can have many causes […]
Read moreInnovative materials in medical devices
Medical devices have become indispensable to everyday life and represent a growing market. Medical device applications range from huge machines (e.g. heart-lung machines) to diagnostic tools such as software and implants, hypodermic needles and mechanical contraceptives, to mention just a few. The compatibility of the materials utilized to create medical devices with the biological surfaces […]
Read moreNanomedicine

Nanomaterials represent important new options for the treament of a variety of diseases. In medicine nanomaterials already are employed for various drugs, diagnostics, and implants. Presently, intense reseach is performed to explore further opportunities for using nanomaterials in the health sector, making nanomedicine an area that – including the public – receives strong attention What […]
Read moreNanobots – reality or fiction?

For centuries, man’s imagination has been inspired by technical developments. Not only Jules Verne (1828-1905) anticipated many technical innovations in his novels, but also in recent times the technical possibilities have inspired the genre of science fiction, especially nanotechnology. When the physicist and Nobel laureate Richard Feynman gave his famous lecture on matter “There is […]
Read moreEducation and Training for Nanotechnology

Nanotechnology is a future labour market, since this branch of industry is quickly growing internationally. In 2013, a total of 1.100 companies in Germany applied nanotechnology in research and development, or were active in marketing commercial goods and services based on nanotechnology [1]. The development and production of nanomaterials is only a part of the […]
Read moreCoatings for Nanomaterials
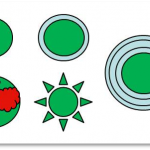
When talking about surface coatings of nanomaterials often terms such as modification, functionalisation or stabilisation are used. This diversity in terms reflects the different motivations for using a coating or for its intended function. Generally, surface coatings of nanomaterials are applied in order to selectively change or influence distinct particle properties. For this purpose, the […]
Read moreCrystal Structures of Nanomaterials
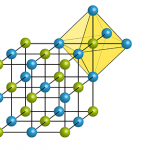
Depending on external conditions such as temperature or pressure, atoms may arrange themselves in various ways in lattice structures. Therefore, for some materials with the same proportions of contained elements, different crystal structures exist. Nanomaterials with different crystal structures may differ in important physicochemical properties (e.g. reactivity or photocatalytic activity). Accordingly, in such cases only […]
Read more >
>