
NanoBioQuant – Quantification of nanomaterials in tissue for regulatory analysis and development of in vitro methods
Nanoparticles (NP) entering the lung via inhalation are largely captured and engulfed by so-called alveolar macrophages. However, small amounts of nanoparticles may by transported by lymphatic fluid and blood flow to remote organs. In previous studies we showed that these nanoparticles are not distributed evenly. Instead, we detected local enrichments, e.g. in liver, spleen and kidney. Whether or not these accumulations mean a risk also for humans exposed to nanoparticles is not known.
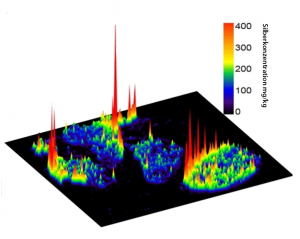
Distribution of silver in a lymph node secondary to administration of silver nanoparticles to the lung. Quantitative spatially resolved image obtained by LA-ICP-MS (Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry). Modified from © Wiemann, M.; Vennemann, A.; Blaske, F.; Sperling, M.; Karst, U. Silver Nanoparticles in the Lung: Toxic Effects and Focal Accumulation of Silver in Remote Organs. Nanomaterials 2017, 7, 441. Licence CC BY 4.0.
Partners of the project NanoBioQuant aim to identify the cell types which accumulate nanoparticles. The concentration of nanoparticles in these cells shall be quantified. To this end, modern mass spectrometry-based imaging methods and immunohistochemistry will be employed to investigate tissue sections mostly from preceding animal studies on short and long term effects of various inhaled nanoparticles. Moreover, novel types of particles of high industrial relevance will be analyzed in parallel.
Information on the type of nanoparticle-laden cells will be used to build up new, innovative cell models, designed to analyze the effects of nanoparticles in a comprehensive manner. In-depth analyses of previous nanoparticle-laden tissues will be conducted, ideally to avoid further animal studies. As one major approach, we will try to adapt the concentrations of nanoparticles in single cells in vitro to the concentrations found in cells in vivo. Therefore, mass spectrometry-based single cell in vitro analytical methods will be established. In addition, we will use novel microscopic approaches and state of the art analyses to unravel effects of nanoparticles on cells.
The analytical detection methods (LA-ICP-MS, ToF-SIMS) will be further developed and refined. The reliable detection and quantification of NP in tissue sections shall be carried out for histopathological sections as used for regulatory toxicology.
By means of a standard operation procedure, the method shall add to existing OECD approved guidelines for risk analysis of inhaled particles and, eventually, may become part of the routine procedure.
The project is led by the IBE R&D Institute for Lung Health gGmbH, Münster, Germany (Prof. Dr. M. Wiemann). Scientific partners come from BASF (Ludwigshafen, Germany) and further institutions located in Münster. The Institute of Inorganic and Analytical Chemistry (Prof. Dr. U. Karst), the Tascon GmbH (Dr. B. Hagenhoff) and the Competence Center Analytics of BASF SE (Dr. O. Hachmöller, Dr. W. Wohlleben) will analyze cells and tissue sections with state of the art mass spectrometry imaging methods. IBE R&D gGmbH and the Toxicology Department of BASF SE (Dr. S. Gröters, Dr. L. Ma.Hock) will provide tissue samples to correlate pathological and analytical findings. The Biomedizinische Technologiezentrum of the Medical Faculty of the University of Münster (Dr. J. Schnekenburger) will investigate NP treated cells and tissues with digital holographic microscopy.
Project Website: www.nanobioquant.de/en/home.html
Grant Number: BMBF - 03XP0213
Duration: 01.04.2019 - 31.03.2022 (extended to 30.09.2022)
Project Lead

Project Partners



https://www.uni-muenster.de/Chemie.ac/

https://www.medizin.uni-muenster.de/en/biomedizinisches-technologiezentrum/about-us.html
Subcontractor


https://www.uk-essen.de/index.php?id=5011&L=1
 >
>