
PLATOX –In vitro and in vivo investigations to generate validated toxicity data of graphene nanoplatelets vs a carbon black reference
The project PLATOX, coordinated by Fraunhofer ITEM, will be performed in cooperation with the partners University of Aveiro (Portugal) and BIO-TOX (Romania). The aim is to generate data on graphene toxicity, as the data so far available is scarce.
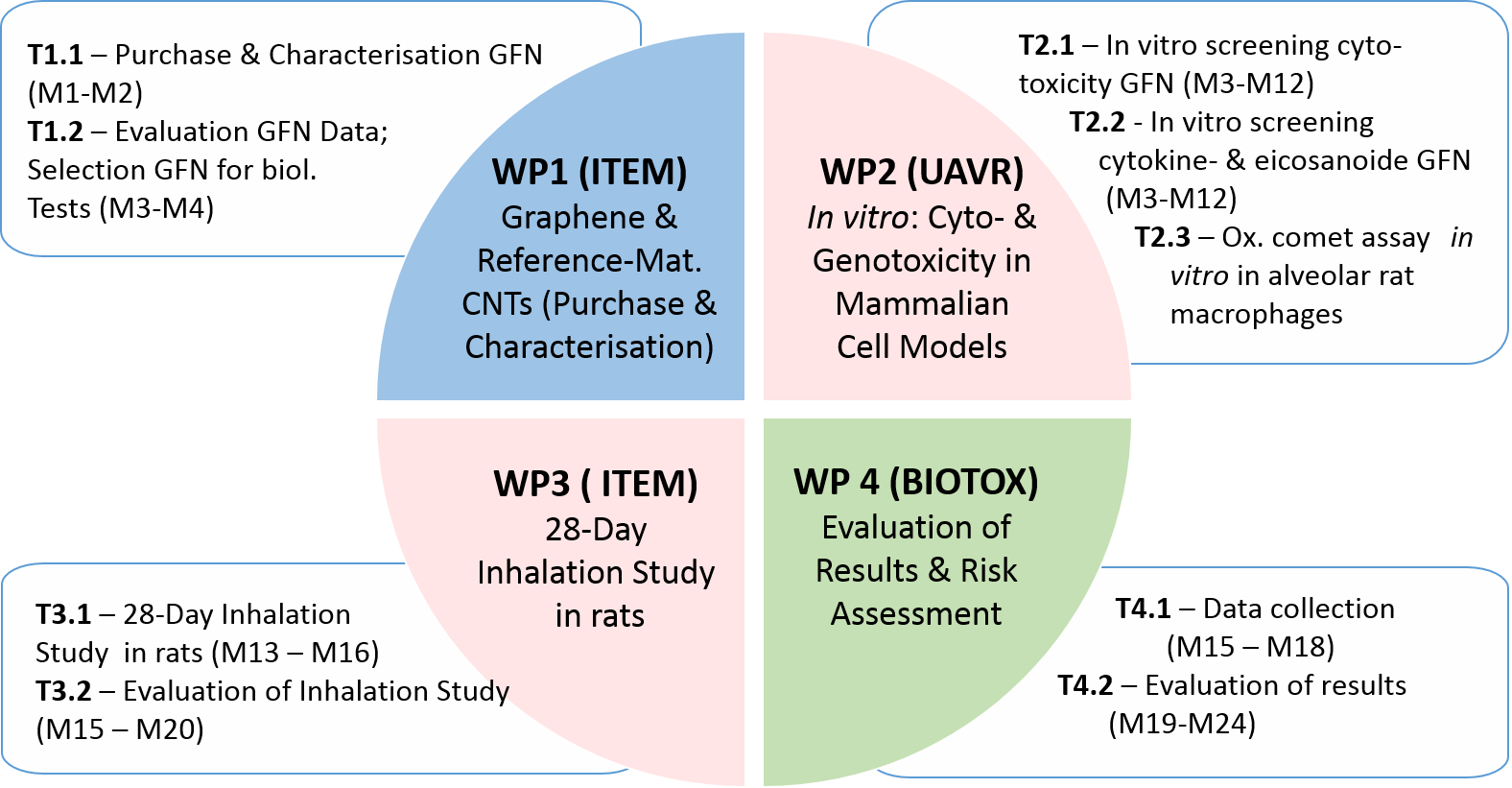
PLATOX Workplan
Graphene nanoplatelets consist of pure carbon, the individual carbon atoms being embedded in a single atomic layer. In practice, a distinction is made between (1) monolayer graphene, (2) few-layer graphene, (3) graphene with up to 10 layers, and (4) graphene with more than 10 layers (known as “graphite”). A graphene layer is about 0.3 nanometres thick, while the length and width of a layer can be 1-20 micrometres. For technical applications, different functionalised graphenes are used, e.g. graphene oxide or carboxylated graphene.
The special particle dimensions of graphene on the one hand result in very attractive technical properties that are being utilised by industry, for example, for flame proofing, plastics reinforcement, in touch screens, or in the medical sector. On the other hand, these dimensions also lead to nanoplatelets having special aerodynamic properties, such as respirability (alveolar region) even at lengths/widths of 5-25 µm. In contrast, for granular dusts/particles the limit for respirability into the deep lung is at a diameter of approx. 4-5 µm.
In an already published 5-day inhalation test (Ma-Hock et al., 2012), the toxic potential exhibited by graphene nanoplatelets was lower than that of multi-walled carbon nanotubes (MWCNT; tangled), but higher than that of technical soot (carbon black). Four carbon compounds could be ranked as follows with regard to lung toxicity: carbon nanotubes (CNT) > graphene nanoplatelets > graphite nanoplatelets > technical soot.
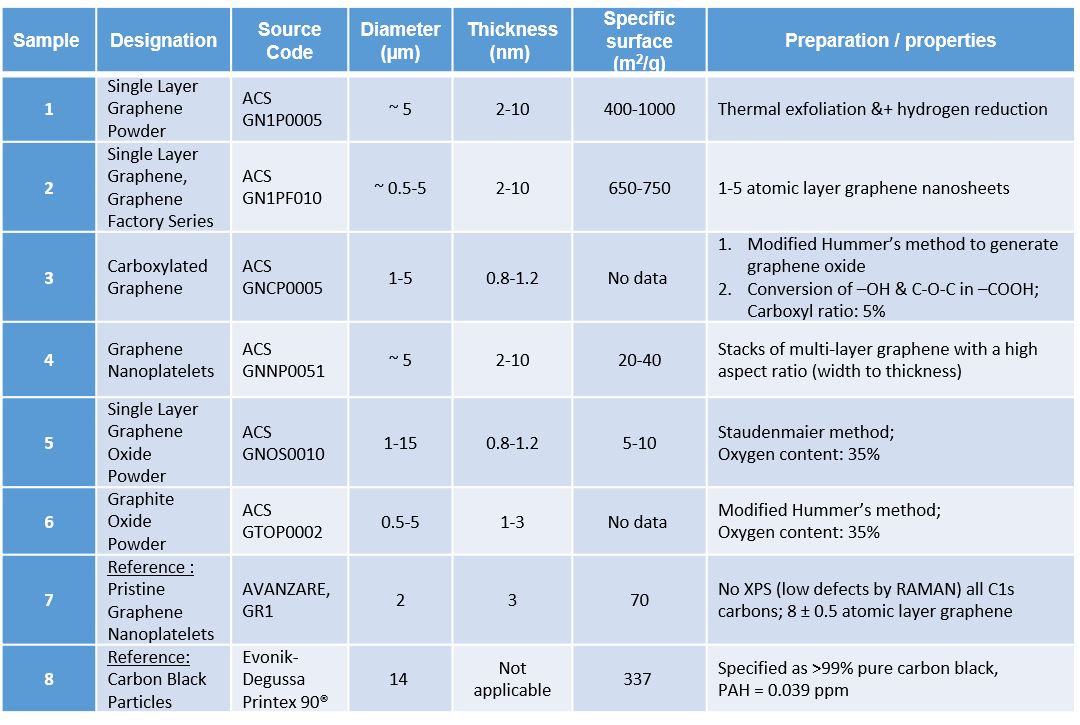
PLATOX Materials overview
Given that particle modifications often result in altered toxicity, different members of the graphene family (GFN) will be compared and toxicological ranking will be performed. The approach consists in determining the biological effects of critical GFN in in-vitro screening assays with a dense network of endpoints. Evaluation of the screening results will allow the GFN with the strongest and weakest biological effects to be determined, and this result will then be subject to in-vivo validation, i.e. in a 28-day inhalation study in rats. Under regulatory aspects, the project PLATOX is aimed at an evaluation of toxico¬lo¬gical data to enable derivation of the DNEL (Derived No-Effect Level), i.e. the level of exposure above which humans should not be exposed (in compliance with REACH).
In a last step, a graphene-specific risk assessment will be performed. The results thus obtained can then be used for calculation of DNEL (Derived No-Effect Level) values and they may become relevant in regulatory contexts.
The results of this project will help improve the basis for risk assessment of graphenes. The information that will be generated is necessary to enhance consumer protection, guarantee occupational safety, and enable establishment of novel products and technologies.
Grant Number: ERA-Net SIINN - FKZ 03XP0007
Duration: 01.12.2015 - 31.03.2018
Project Lead
Project Partners

 >
>