 >
CaNTser
>
CaNTser
CaNTser
CaNTser – Investigating the toxic potential of carbon nanotubes after long-term inhalation
Research in the last few years has clearly demonstrated that carbon nanotubes have different toxic properties depending the individual CNT structure. Carbon nanotubes with a short or convoluted morphology cause only minor toxic effects, which correspond well to particles without specific toxicity. In contrast, results of the BMBF-funded project CarboTox (2010 – 2013) indicated that long and fibrous CNTs could cause tumour-inducing effects similar to asbestos. Since this hypothesis might have far-reaching consequences for humans, a scientific investigation is urgently needed to either prove or disprove that carbon nanotubes are able to initiate asbestos-like cancer formation in humans upon inhalation
The main objective of the project CaNTser is therefore to examine the tumour-causing effects of fibre-like CNTs after inhalation, to detect a possible mechanism of action and possibly derive a threshold value.
Further investigations have shown that the toxicity and probably also the tumour formation of carbon nanotubes depends strongly on the different CNT modifications (length, diameter, curvature, surface properties)as well as on the separating / caking properties. Therefore, short-term screening methods are applied to determine in vitro and in vivo the characteristics of those carbon nanotubes, which cause, potentiate or weaken the toxic and thereon-based carcinogenic effects
Another important goal of the CaNTser project is to overcome the challenge to specifically control the synthesis of carbon nanotubes in order to generate either straight or curved carbon nanotubes, which are required for the different biological assays and endpoints. This will be achieved by controlling the defect density of the CNTs, which requires an accurate and reproducible procedure of the synthesis process.
NanoBEL
NanoBEL – Biological Elimination of Complex Diagnostic Nanoparticles

NanoBEL medical applications (c) NanoBEL Consortium
Nanotechnology is one of the key technologies of the 21st century that developed from a basic research to a worldwide important discipline in the last years with an enormous importance in life sciences and medicine. Magnetic nanoparticles play an important role in diagnostic imaging for prevention, therapeutic monitoring and therapy.
Whereas the toxicological effects of acute nanoparticle expositions were widely described in the last years, long-term risks depending on the structure of the nanomaterials and the disease state of the patients have not been systematically investigated.
NanoBEL is focused on the risk assessment of the long-term effects of magnetic nanoparticle exposition particularly after repeated administrations, as well as the role of degradation and elimination in the life-cycle of the nanoparticles in diseases like cancer and inflammation. Innovative magnetic nanoparticle formulations with a high relevance for future diagnostic applications are taken into consideration. Beside the development and optimization of magnetic nanoparticles,
NanoBEL contributes to the development and validation of new alternatives to animal-based methods (cell cultures, hen’s egg models) to be applied in an integrated safety assessment of the long-term administration of nanoparticles. In close collaboration with DaNa2.0, the systematic collection of the data in a data base accomplish the categorization of nanomaterials, the identification of relevant endpoints, and the risk management.
ProCycle
ProCycle – Analysis and toxicological evaluation of dusts from recycling and recycling processes of nanocomposites and strategies for risk minimisation
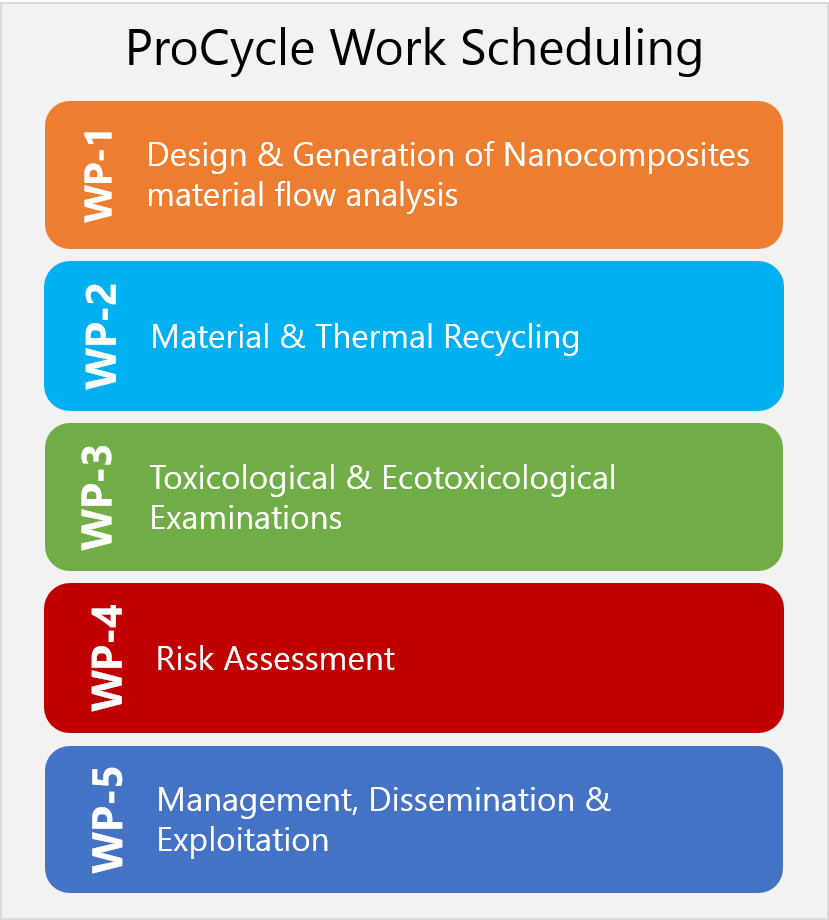
ProCycle Workplan
ProCycle investigates and evaluates the toxicological and ecotoxicological relevance of dust and gas emissions that occur during the recycling and thermal utilisation of nanocomposites (NC). This includes interpreting the results for REACH registration processes. Methods need to be developed for a reliable on-the-spot characterisation of the dusts and possibly occurring gases during comminuting or thermal recycling of nanocomposites, especially in order to obtain realistic emission values when using exposition modules for toxicology.
The aim is to reduce the risk potential of the dusts resulting from the recycling processes and of the gases produced during thermal disposal.
For this, a proposal for a continuous investigation and evaluation methodology for the application of nanomaterials in plastics and for material and thermal recovery will be developed.
In joining partners with competences in the areas of compounding, comminution, combustion, measurement technology, toxicology, and REACH assessment the project ProCycle offers a research and development approach which covers the entire process chain.
nanoGRAVUR
nanoGRAVUR : nanostructured materials – grouping for occupational health, consumer and environmental protection and risk mitigation
Nanotechnology offers numerous new applications in various areas of industry (e.g., in the chemical industry, electrical engineering, and medical engineering). The specific challenge lies in the optimal use of the potentials of these partially new technologies and, at the same time, in their responsible handling.
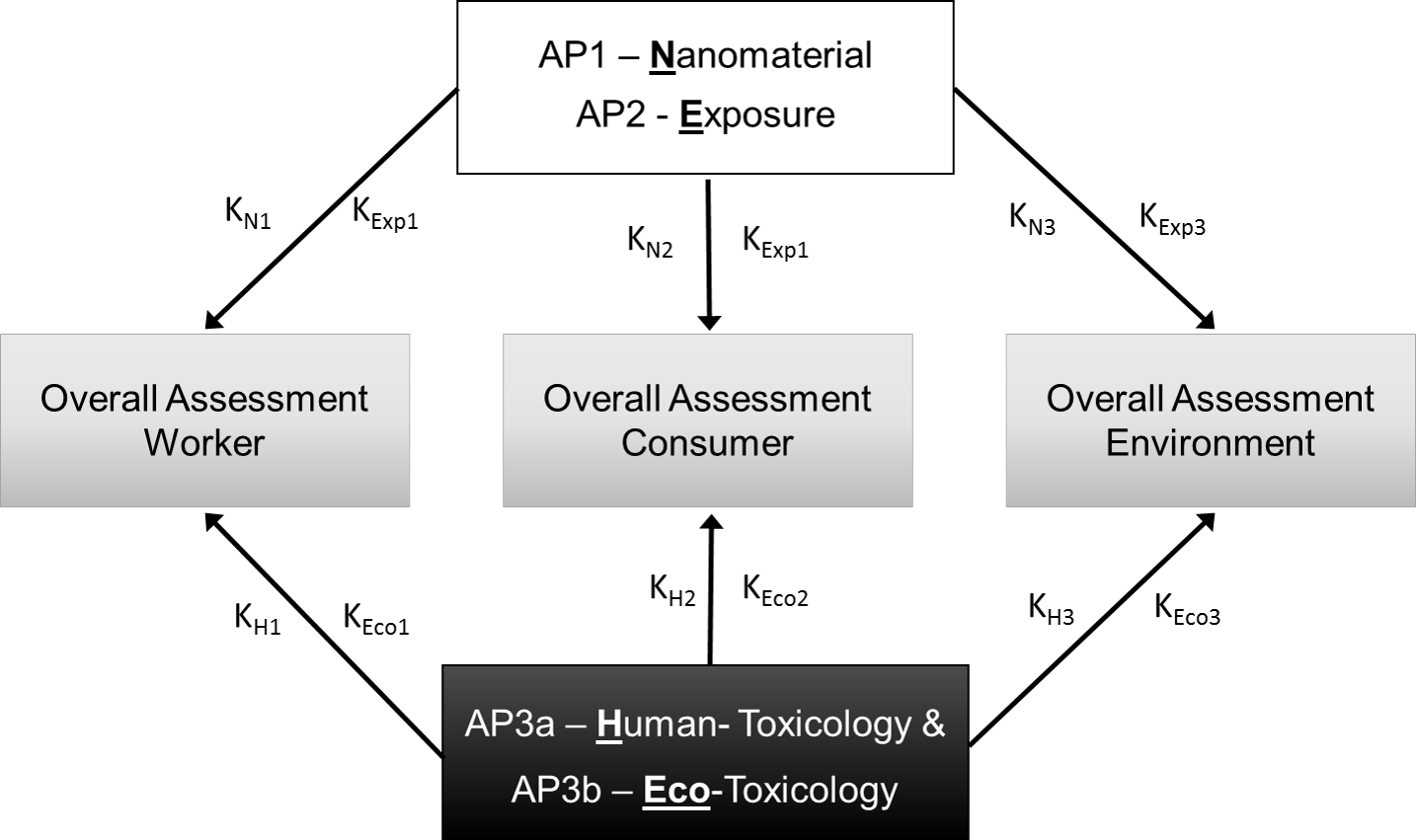
Scheme for the derivation of criteria catalogues (K) for different protected assets based on the risk-determining factors nanomaterial properties (N) release / exposure (Exp) and hazard (H and Eco).
In view of the large variety of existing synthetic nanomaterials, some of which have been used for decades in conventional products and most of which, besides, are available in numerous modifications (different sizes, shapes, chemical composition, and surface functionalisation), the effort of analysing their behaviour and effects while observing specific regulatory requirements is tremendous. Regarding the variability of possible effects, it is, in addition, impossible, to assess the potential risk for each individual nanomaterial.
The current state of knowledge on the hazard of nanomaterials towards protected assets is so complex that the nanoGRAVUR project made it its central objective to develop different criteria catalogues for a grouping of nanomaterials according to the respective potentials for exposure, hazard and risk. The grouping approach, which so far has been used only in special cases (e.g. for fibres), can, among other things be used in areas such as occupational health and safety, product identification, and regulation, which presently still must rely on individual case studies.
Project Website (in German): www.iuta.de/nanogravur/
nanoCOLT
nanoCOLT – Long-term effect of modified carbon black nanoparticles on healthy and damaged lungs
Based on the previous project CarbonBlack, the joint research project nanoCOLT investigated the interrelationships between material properties of manufactured carbon black nanoparticles (CBNP) and potential lung damaging effects in a low-dose range. A particular focus of the project nanoCOLT was to test first, if long-term CBNP-exposure of healthy lungs results in any histological and physiological alterations. Second, nanoCOLT aimed at testing if pre-damaged (diseased) lungs would respond more sensitive than healthy lungs to an exposure with carbon black nanoparticles. Further, the project sought to identify cell and tissue culture systems, which were suitable to predict potential effects of carbon black nanoparticles in humans. The results obtained from these in-vitro and ex-vivo test systems were finally verified in an in-vivo inhalation exposure experiment in rats according to OECD guideline 413.
Various in-vitro and ex-vivo test systems, partly established in the previous project CarbonBlack such as e.g., explant models of the murine trachea and of intra-pulmonary airways as well as human precision cut lung slices, were refined and novel test systems such as a particle-induced cell migration assay (PICMA) and an air-liquid-interface epithelial cell culture model were tested for their suitability as toxicity assays. In the in-vitro and ex-vivo systems, the carbon black nanoparticles to be tested were applied at concentrations of 1, 10 and 30 μg/ml, whereas in the in-vivo animal experiments a dose of 70 μg carbon black nanoparticles per animal were applied either once or seven times at an interval of two weeks.
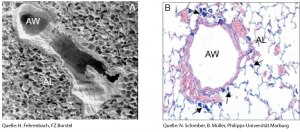
Images of the lung taken with (A) a Scanning Electron Microscope or (B) light microscope showing the airways (AW) and surrounding alveoli (AL). Arrows indicate inhaled agglomerates of carbon black nanoparticles (CBNP). © H. Fehrenbach / FZ Borstel; © N. Schreiber und B. Müller / Universität Marburg
Based on our studies in the ex-vivo and in-vivo animal models, our research network concludes that the tested tailor-made carbon black nanoparticles exhibited only a low degree of acute toxicity both in healthy lungs as well as in lungs pre-damaged by exposure to nitrogen dioxide (NO2) or experimentally induced allergic asthma. The low-level effects observed are particularly determined by their surface chemistry. Repetitive exposures as model of a long-term exposure also resulted in only low-level effects of the test carbon black nanoparticles in the ex-vivo and in-vivo animal experiments. The in-vivo inhalation exposure study was performed using Printex®90 as reference particle and acetylene soot and Printex®90 coated with benzo[a]pyren as test carbon black nanoparticles. The sub-chronic exposure acetylene soot induced a stronger inflammatory response in rats than exposure with Printex®90 or Printex®90 coated with benzo[a]pyren.
A wide spectrum of methods was used to characterize the material properties of the modified carbon black nanoparticles with a particular focus on the blockade of surface functions of the carbon black nanoparticles due to an interaction with proteins present in the biologic environment. To study this aspect, a wide variety of spectroscopic method were applied. There were no indications that changes in secondary structural elements could act as modulators of the biologic activity of the tested carbon black nanoparticles. The reasons for the overall low-level acute toxicity of the carbon black nanoparticles are apparently due to a relatively strong interaction between the proteins and the surface groups of the carbon black nanoparticles. Through this, the toxic effects of surface groups or molecules on the surface could be shielded or reduced, respectively.
The ex-vivo studies in human precision-cut lung slices revealed that also in the human test system there was no direct cell-damaging effect of the test carbon black nanoparticles on healthy and diseased (pre-damaged) lungs. The carbon black nanoparticles rather induced initial inflammatory processes in healthy human tissues that are characteristic of physiologic reactions on noxae and which can be seen as protective mechanisms. Notably, no such inflammatory reactions to be seen as physiologic protective mechanisms were observed in tissues of diseased lungs (pulmonary fibrosis) exhibiting marked histopathologic alterations. The results obtained in the human ex-vivo test system are largely congruent with the findings from the ex-vivo and in-vivo animal experiments.
In summary, the joint research network nanoCOLT finally concludes that, on the basis of a variety of experiments using in-vitro, ex-vivo and in-vivo test system in mouse, rat and human cells and tissues, respectively, the acute and sub-chronic toxicity of the test carbon black nanoparticles in the low-dose range can be ranked as low and that the effects are primarily determined by the surface chemistry. Whether the absence of inflammatory reactions seen as physiologic protective mechanisms, which was observed in the human ex-vivo test system when using histopathological strongly altered tissues, will have a negative impact on the underlying lung disease during chronic exposure and increasing persistence of the carbon black nanoparticles in the lung, has to be addressed in future studies.