 >
Spotlight February 2022: Probabilistic risk assessment – the keystone for the future of toxicology
>
Spotlight February 2022: Probabilistic risk assessment – the keystone for the future of toxicology
The basics of toxicology are constantly being reconsidered, and the approach to risk assessment is therefore constantly being put to the test, because, as William Osler is cited in this publication, “Medicine (toxicology) is a science of uncertainty and an art of probability“.
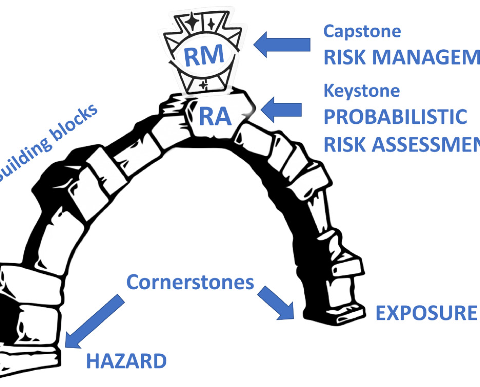
In this recent paper, the team around Thomas Hartung (Johns-Hopkins University/University of Konstanz) has shown that for improved toxicology we should rather work with a “Probabilistic Risk Assessment” approach. This is also or especially important for new materials, because with these there are particularly often gaps in knowledge, uncertainties in risk assessment due to conflicting data and the most diverse hypotheses and strategies of the various stakeholders. In the publication, various models are presented that are applicable for this type of risk assessment and for some of which corresponding software is also available to perform calculations for the respective exposure scenarios. In the examples for this approach, a paper by Jacobs et al. (1) is also cited here, who had applied the case to silica in food. They concluded that after taking all uncertainties into account and using all available data, the margin of safety has not yet been exceeded by far using silica in various food products. In 2017, an international group of experts applied this method to Titanium dioxide in seven different exposure scenarios and concluded no increased risk to humans, as the probability of exceeding the safety limits is vanishingly small (2).
The suggested approach by Johns Hopkins University is thus a good indication to adopt this method in order to be able to make a reasonable risk assessment for new, innovative materials even in the presence of uncertaintie.
Further literature:
- Jacobs, R., van der Voet, H., and Ter Braak, C.J. (2015). Integrated probabilistic risk assessment for nanoparticles: the case of nanosilica in food. J Nanopart Res 17, 251
- Tsang, M.P., Hristozov, D., Zabeo, A., Koivisto, A.J., Jensen, A.C.O., Jensen, K.A., Pang, C., Marcomini, A., and Sonnemann, G. (2017). Probabilistic risk assessment of emerging materials: case study of titanium dioxide nanoparticles. Nanotoxicology 11, 558-568
Original publication:
Maertens, A., Golden, E., Luechtefeld, T.H., Hoffmann, S., Tsaioun, K., and Hartung, T. (2022). Probabilistic risk assessment – the keystone for the future of toxicology. ALTEX 39, 3-29
Weitere Spotlights
Spotlight September: A methodology for the automatic evaluation of data quality and completeness of nanomaterials for risk assessment purposes
This paper describes a method for automatically assessing the quality and completeness of nanosafety data for the purpose of risk assessment. Steps to develop the methodology for assessing data completeness and the methodology for assessing quality are presented. The methodology is tailored to physicochemical and hazard (meta) data, but can also be configured with appropriate […]
Read moreSpotlight September 2023: Fishing for raw materials with proteins
The so-called rare earth elements such as neodymium, dysprosium or cerium are elements that are of great importance for the energy transition; among others they serve as components of magnets in generators for electric power generation, act as luminescent materials in energy-saving lamps or as part of the car exhaust catalytic converter. The global production […]
Read moreSpotlight April 2023: Recycling rare earths – bacteria assist in the circular economy
Rare earths are important components of wind turbines, catalytic converters, fibre optic cables and plasma screens. Since the 17 metals grouped under this term are indispensable for modern technologies, demand and costs are constantly rising. The occurrence of productive mining sites is limited and the production is often costly and environmentally harmful. The advantages of […]
Read moreSpotlight June 2023: New catalytic process for recovering important materials from composites in a single process
Previously virtually impossible and a huge problem: fibre-reinforced resin composites (epoxides) were not recyclable, and wind turbine rotor blades, for example, add up to a waste pile of 43 million tons by 2050. Researchers have now taken an important first step in “reprocessing” these composites and catalytically dissolving them so that the carbon fibres and […]
Read more


