>
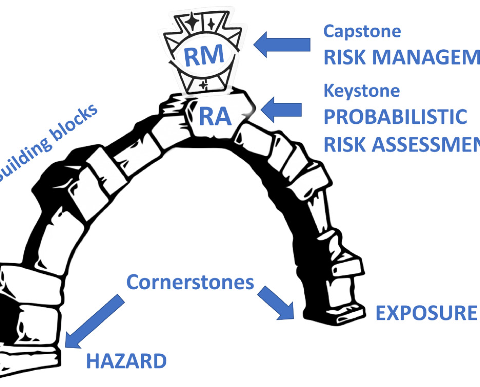
Spotlight February 2022: Probabilistic risk assessment – the keystone for the future of toxicology
>
Spotlight February 2022: Probabilistic risk assessment – the keystone for the future of toxicology
The basics of toxicology are constantly being reconsidered, and the approach to risk assessment is therefore constantly being put to the test, because, as William Osler is cited in this publication, “Medicine (toxicology) is a science of uncertainty and an art of probability“.
In this recent paper, the team around Thomas Hartung (Johns-Hopkins University/University of Konstanz) has shown that for improved toxicology we should rather work with a “Probabilistic Risk Assessment” approach. This is also or especially important for new materials, because with these there are particularly often gaps in knowledge, uncertainties in risk assessment due to conflicting data and the most diverse hypotheses and strategies of the various stakeholders. In the publication, various models are presented that are applicable for this type of risk assessment and for some of which corresponding software is also available to perform calculations for the respective exposure scenarios. In the examples for this approach, a paper by Jacobs et al. (1) is also cited here, who had applied the case to silica in food. They concluded that after taking all uncertainties into account and using all available data, the margin of safety has not yet been exceeded by far using silica in various food products. In 2017, an international group of experts applied this method to Titanium dioxide in seven different exposure scenarios and concluded no increased risk to humans, as the probability of exceeding the safety limits is vanishingly small (2).
The suggested approach by Johns Hopkins University is thus a good indication to adopt this method in order to be able to make a reasonable risk assessment for new, innovative materials even in the presence of uncertaintie.
Further literature:
- Jacobs, R., van der Voet, H., and Ter Braak, C.J. (2015). Integrated probabilistic risk assessment for nanoparticles: the case of nanosilica in food. J Nanopart Res 17, 251
- Tsang, M.P., Hristozov, D., Zabeo, A., Koivisto, A.J., Jensen, A.C.O., Jensen, K.A., Pang, C., Marcomini, A., and Sonnemann, G. (2017). Probabilistic risk assessment of emerging materials: case study of titanium dioxide nanoparticles. Nanotoxicology 11, 558-568
Original publication:
Maertens, A., Golden, E., Luechtefeld, T.H., Hoffmann, S., Tsaioun, K., and Hartung, T. (2022). Probabilistic risk assessment – the keystone for the future of toxicology. ALTEX 39, 3-29
Weitere Spotlights
Spotlight October 2023: Improved hydrogen production through novel catalyst made of three metals
Hydrogen is one of the important energy carriers of the future when it comes to climate-relevant energy supply. For example, surplus electricity from wind turbines or solar plants can be converted into hydrogen, allowing the otherwise unused energy to be stored for longer periods. This hydrogen can be used to power trucks and buses for […]
Read moreSpotlight March 2022: Safe Materials from Scratch – Safe-by-Design-Concept in action
In recent decades, German research on nanomaterials and new, innovative materials has been widely expanded by material safety aspects. European initiatives also pay significant attention to this: both the European Union (EU) Green Deal, and the Chemicals Strategy for Sustainability (CSS) aim to create a sustainable, climate-neutral economy with sustainable and safe chemicals and products, […]
Read moreSpotlight May 2023: Dual energy – edible batteries
An Italian research group reports on edible batteries that supply electric current and can be digested as food, thus providing energy a second time. What sounds funny at first has a serious background, because in medicine, power sources are needed that could be transported through the digestive tract and possibly remain in the body unintentionally, […]
Read moreSpotlight September 2021: Wood, the raw material of the future?
One of the greatest challenges facing humanity is to produce clean drinking water under the given circumstances of global warming, population growth and increasing littering. In September, we would like to present a review article that believes one approach to solve this problem is the use of nanoscale wood. In the review, “Advanced Nanowood Materials […]
Read more