After their release, innovative materials or nanomaterials are not only distributed in the air, soil or water (see Basic Information: How are innovative materials (e.g. nanomaterials) released?) but can also be changed, i.e. they undergo transformation processes (so-called transformation). These are processes in which the materials interact with the environment and are changed. Transformation can be limited to the particle surface or affect the particles as a whole. These processes can affect the behaviour and distribution of the innovative materials in the environment, and can be divided into chemical, physical, or biological processes. In chemical reactions, the innovative materials or nanomaterials form a bond with other substances from the environment (e.g. oxygen from the air, ‘rusting’). If the innovative materials remain in their chemical structure and attach to other substances (e.g. adsorption) this is referred to as physical transformation. All changes in materials caused by biological systems (e.g. by bacteria) are referred to as biological transformation.
Whether and how such processes occur depends on the environmental compartment (air, water, soil) and the conditions prevailing there. In air, physical transformation occurs predominantly, i.e. the accumulation of different particles (heteroaggregation) or the aggregation of identical particles (homoaggregation) and the attachment to surfaces. In the soil, mainly chemical and biological transformations take place due to bacteria and fungi present. All three processes (physical, chemical and biological transformation) can be observed in water. However, the transformation processes that take place depend not only on the environmental compartment, but also on the particular innovative material. For example, some materials tend to dissolve, i.e. they form a compound with another substance and are then usually dissolved in water (e.g. silver, copper). Other materials are known to attach rapidly to other particles and then settle to the bottom (sediment; e.g. iron or iron oxide particles).
Transformation processes can rarely be observed directly. Because of the difficulty of detection, modelling is needed to simulate transformation behaviour as a function of different properties of the innovative materials (e.g. reactivity). These models serve to understand the distribution and interaction of innovative materials in and with the environment and are thus also a basis for toxicological risk assessment.
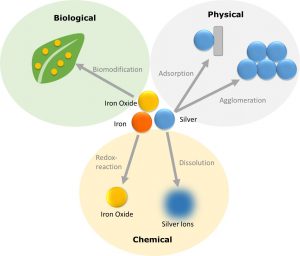
Overview of the transformation processes to which innovative materials are subject ©DaNa
Chemical transformation processes
In chemical transformation processes, the components of innovative materials are converted into other forms (e.g. silver into silver sulfide) as a result of chemical reactions. Chemical transformation processes depend on various factors. One decisive factor is light, which can trigger redox reactions (i.e. the parallel oxidation and reduction of different substances). Radicals (uncharged, reactive particles) are formed by this so-called photocatalysis. Another factor in chemical transformation processes is the presence of oxygen. An example of this is the rusting of metals. Similarly, carbon-based innovative materials can be oxidized at the surface.
Due to the good conductivity of ions and electrons, many redox reactions take place in aqueous environments (e.g. rusting of iron nanoparticles). Another decisive factor in water is solubility e.g. in the case of nanosilver particles. These slowly release silver ions into the environment, thus dissolving. Depending on the environment and further reaction partners contained in the water (here e.g. sulfur ions), nanoparticles can be formed again, as the poorly soluble silver sulfide is formed and precipitates, i.e. forms a solid precipitate (precipitation). Thus, silver particles become the silver sulfide particles by chemical transformation.
Physical transformation processes
Physical transformation processes mainly include addition and deposition processes. Important processes here are homo- and heteroagglomeration, i.e. the aggregation of nanoparticles and other particles into larger complexes. One consequence of this is sedimentation, in which innovative materials settle to the bottom in aqueous environments. The same applies to the attachment to various structures, e.g. stones or organisms, and the detachment of the innovative materials from surfaces again (adsorption/desorption).
Biological transformation processes
Animal and plant life also have an important influence. Microorganisms can not only decompose organic substances, which is also called biodegradation. For example, fungi and bacteria can degrade carbonaceous innovative materials or dissolve metallic particles. Biomodification, which involves the incorporation of nanoparticles into more complex structures (e.g. incorporation of iron oxide nanoparticles into plant leaves), has also been demonstrated in plants and animals.
Transformation processes are very diverse and complex processes that can be simplified into chemical, biological and physical processes. During these processes, the properties of the innovative materials and thus also their distribution in the environment can change. For a valid risk assessment of innovative materials, a detailed consideration of all three transformation processes is necessary.
Literature
Abbas, Q et al. (2020). Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review. Environ Int, 138 105646.
 >
>