 >
nanoIndEx
>
nanoIndEx
nanoIndEx
nanoIndEx – Assessment of Individual Exposure to manufactured nanomaterials by means of personal monitors and samplers
Exposure to airborne nanomaterials needs to be measured in the personal breathing zone using nano-specific personal samplers or monitors. Such personal instruments have only become available over the recent years.
The main objectives of the nanoIndEx project were hence to scrutinize the possibilities and limitations of these novel instruments in both laboratory and field studies and then to generate reliable data regarding the personal exposure to airborne nanomaterials in real workplaces. nanoIndEx therefore studied the accuracy and comparability of the samplers and monitors, which was found to be typically around ± 30 % or better for compact or agglomerated particles. Dedicated studies on their field applicability showed that this accuracy and comparability is still applicable under real field conditions and that all tested instruments are robust and ready for field use.
However, the choice of sampling tube material needs to be carefully considered, because some polymer types were found to induce drastic artefacts when connected to monitoring devices. It was furthermore found that none of the applied monitors could be used to measure exposure to fibrous aerosols, e.g. containing carbon nanotubes (CNTs). Instead, the development of a sampling and data evaluation routine for fibres was initiated.
Standard Operation Procedures (SOPs) regarding the proper use of the personal monitors and samplers and the performance of field studies have been written and shared via the project website www.nanoindex.eu. Numerous field studies have been conducted and a data collection and evaluation protocol has been developed. All applicable data have been uploaded to the international Nano Exposure and Contextual Information Database (NECID).
One main objective of the project was to widely distribute the project’s findings. Consequently, a 49- page Guidance Document “Assessment of Personal Exposure to Airborne Nanomaterials – a guidance document, NanoIndEx Consortium 2016” on the assessment of personal exposure to airborne nanomaterials has been written, printed and disseminated. It is also available on the project website. In addition, a large number of peer reviewed papers and book chapters have been (or will be) published and numerous presentations were (and will continue to be) given at various occasions to inform among others exposure scientists, occupational hygienists and workplace practitioners about the project results.
NanoCare
A German Initiative on the Health Aspects of Synthetic Nanoparticles:
Establishing an Information- and Knowledge-Base for Innovative Material Research
The NanoCare project (running from 2006 until 2009) aimed at broadening the knowledge about synthetic nanomaterials with regard to the potential impacts of nanomaterials on human health. New innovative applications and measurement methods were developed for a sustainable and caretaking use of chemical nanotechnology.
Data created within NanoCare were interpreted together with additional information from literature and then published by the “knowledgebase”. This publication of nanotechnology safety aspects satisfied increasing information needs of the public. Furthermore, the results were presented to and discussed with the interested public, politicians and NGOs at dialogue events. Partners from industry, universities and research institutes were contributing their expertise to this partnership.
nanoGEM
nanoGEM – Nanostructured Materials – health, exposure and material properties
The NanoGEM research project, funded by the BMBF and industry, was an integrative interdisciplinary project that carried out various research activities on nanosafety, exposure, risk, and toxicology in a combined effort of academia, authorities and industry. The results and findings obtained within NanoGEM were published in international journals (see NanoGEM publications) and included in the international harmonisation process and the public dialogue. In addition, practical safety proposals and standard operating procedures (SOP) were developed and published.
The specific research activities were divided into seven work packages. Knowledge of the basic properties of nanomaterials (NM) plays an essential role in the investigation and assessment of safety issues relevant to humans and the environment.
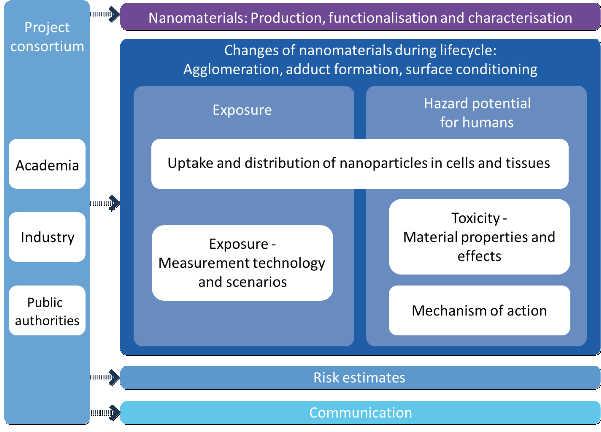
NanoGEM Project Description
The work packages “production, functionalisation and characterisation of nanomaterials” mainly dealt with the development and allocation of new materials with modified basic properties, and modified surfaces in particular. The surfaces of selected nanomaterials such as silver (Ag), silicon dioxide (SiO2), and zirconium dioxide (ZrO2) were specifically modified and thoroughly characterised in water and relevant biological media together with other reference and comparison materials. Labelling of silicon dioxide and titanium dioxide (TiO2) nanoparticles with the fluorescent marker FITC (fluoresceine isothiocyanate) allowed for identification, localisation and mobility tracking of these nanoparticles in different media.
Studies within the work package “exposure – measuring techniques and scenarios” investigated the basic principles required for exposure assessment, release mechanisms and measuring strategies. The results of the main questions like “Is it possible to reproduce and simulate certain release scenarios in the laboratory in a comparable way?” and “Which measuring techniques and strategies can be used for exposure studies?” were integrated into a testing strategy which meanwhile has been included in the OECD program of the Working Party of Manufactured Nanomaterials (WPMN).
In a special work package called “cross-cutting issues: nanomaterial modifications”, modifications of the properties of nanomaterials over the entire life cycles were analysed placing emphasis on the lungs as the most health-relevant routes of entry of nanomaterials into the body.
Surface functionalisation had an essential influence on the adsorption behaviour of the lung surfactant lipids’ towards the nanomaterials such that after attachment of the different components (lipids, proteins, organic compounds), there was no direct contact between the surface of the nanomaterials and the surrounding medium. In the alveoli or at the air-epithelium interface, the once stable suspensions of the respective nanoparticles (SiO2 FITC, adsorptively labelled ZrO2) agglomerated over the entire observation period (30 – 180 min) resulting from the interaction with the proteins of the lung alveoli.
Within the topic “nanoparticle toxicology: material properties and effects”, in vitro and in vivo analyses were carried out on sensitised mice, among others. Whereas, in both scenarios, the unmodified silicon dioxide nanomaterials were found to cause the strongest effects, no significant reactions were observed in the amino- and phosphate-functionalised SiO2 materials. The risk-related studies revealed that the risk potential of a substance can be changed through surface modification and that this may lead to a better matrix integration.
For risk assessment, various data on chemical safety, occupational health and safety, and consumer protection were compiled and evaluated. For the derivation of the risk assessment of nanomaterials within the context of chemical safety legislation specific data on the endpoints related to the three selected nanoscale materials silicon dioxide, silver, and zirconium dioxide were collected and analysed. The evaluation revealed that, in view of the fact that no solely nano-specific modes of action have been identified so far and that conventional risk assessment principles can also be applied to nanomaterials.

Wohlleben W., Kuhlbusch T.A.J., Schnekenburger J., Lehr C.M. (2015). Safety of Nanomaterials along Their Lifecycle: Release, Exposure, and Human Hazards. Taylor & Francis Inc, CRC Press, pp. 472. ISBN: 9781466567863.