Since the discovery of carbon nanotubes (CNTs) in 1991, this group of carbon molecules has been considered as a wonder material due to some interesting properties. They are stronger than steel but very light in weight. Depending on the production method, the nanotubes can be conducting in nature, semi-conducting or even isolating, which makes this material very interesting for the electronic industry. But up to now production volumes of carbon nanotubes are still very low.

Hightech Bicycle © Robert Neumann / fotolia.com
How can I come into contact with this material?
Since carbon nanotubes are produced in very low amounts and since their application is limited, the chance of direct contact of humans with these nanotubes is low. Carbon nanotubes are used in some composite materials or electronic components. Since there is no release of nanotubes from these products under normal circumstances there exists only a small chance of getting into contact with once the products are disposed of at the end of the products’ life cycle.
Is there any risk from this material to humans and the environment?
Due to their long and fibre-like structure carbon nanotubes may elicit fibre-like (adverse) biological effects in the lung which is why they have been thoroughly investigated from the beginning. A general principle is that fibre-like materials can cause lung problems if the fibres are longer than 15-20 micrometres (one fourth to one third of a diameter of a human hair). Fibres with such length are well-known to cause adverse effects in the lung inducing lung inflammation and lung tumours (asbestosis-like disease). This principle also applies to carbon nanotubes with a similar length and rigidity. The clearance mechanisms of the lung can’t cope with such long and stiff materials causing a permanent inflammation of the affected tissue(s) which may then lead to tumour formation after a prolonged period of time (20 to 30 years).
Conclusion
Due to the low production volume of carbon nanotubes there is unlikely to be significant negative influences for humans and the environment. The situation may change however if the worldwide production volumes increase significantly due to new applications and new products containing carbon nanotubes.
By the way…
In 2013 the Bayer Group closed its production site for carbon nanotubes due to economic reasons.
Properties and Applications
About 30 years ago with the discovery of the football-shaped carbon nanoparticles, called fullerenes, a new development in carbon research began. It will lead to lasting effects on many areas in technology and everyday life. Once their principle of design had been uncovered more variations of the original molecules were developed. As a result of these developments the "elongated" form of fullerenes, called carbon nanotubes (CNTs), were discovered. Beside graphite, diamond, fullerenes and graphene CNTs are a further modification of the element carbon. In addition, CNTs may be one of the most cited nanomaterials.
Due to their unique properties, e.g. CNTs are very stable, and potential applications of CNTs, they are of interest for research and industry. The tensile strength of a multi-walled carbon nanotube was determined to be 63 GPa, which is about 50 times the amount that of steel, but at a much lower weight. Moreover, CNTs may also be electrically insulating, semiconducting or of metallic conductance, depending on the way they are manufactured.
![Overview on different carbon nanotube structures: single-walled (SWCNT), double-walled (DWCNT), multi-walled (MWCNT) and possible surface modifications. © Adapted with permission from Jackson, P et al. (2013). Bioaccumulation and ecotoxicity of carbon nanotubes. Chem Cent J, 7(1): 154. Open Access article [CC-BY-2.0 (http://creativecommons.org/licenses/by/2.0)]. Overview on different carbon nanotube structures: single-walled (SWCNT), double-walled (DWCNT), multi-walled (MWCNT) and possible surface modifications.](https://nanopartikel.info/wp-content/uploads/2020/11/CNT-Jackson-2013-EN.png)
Overview on different carbon nanotube structures: single-walled (SWCNT), double-walled (DWCNT), multi-walled (MWCNT) and possible surface modifications. © Adapted with permission from Jackson, P et al. (2013). Bioaccumulation and ecotoxicity of carbon nanotubes. Chem Cent J, 7(1): 154. Open Access article [CC-BY-2.0 (http://creativecommons.org/licenses/by/2.0)] .
Thus, they might be useful for a wide range of applications. Nearly all industries hope for innovative high-tech applications of CNTs. Examples of such applications are transistors made of nanotubes, nanotube memories, nanotubes to improve plastics and their use in measurement technology. By far, these are not all the fields of applications which may be available. All key industries of our modern societies have possible applications of CNTs or already offer products on the market. For example CNTs are used as additives to various plastics in electronics, in the automotive industry, for lightweight construction or for the production of sports equipment.
CNTs may be useful to contribute in a meaningful manner to the challenges of the "Energiewende" in Germany, the transition from nuclear and fossil-fuel energy to renewable energy. This may be achieved by improving batteries, enforcing rotor blades of wind turbines or by applications in solar- and fuel-cells, but also in construction chemicals to create high performance concrete. These potential fields of application could be ready for use in real products in the immediate future.
The variety of possible applications exhibits a considerable economic potential. Therefore, in Germany a research alliance of more than 90 well-known partners from research and industry had been established in the Innovation Alliance Carbon Nanotubes (2008-2014, Inno.CNT) to focus on the development of CNTs and CNT-derived products. This Alliance, which is endowed with 90 million Euros, is funded 50% by the federal government.
Carbon nanotubes are divided into two classes, which consist of single-walled CNTs (SWCNT) with a diameter of less than 5 nm on the one hand and multi-walled CNTs (MWCNT) with diameters up to and more than 100nm on the other hand. Though they are interesting due to their economic opportunities CNTs are also investigated with no direct economic applications in mind. In addition, similar to other carbon nanoparticles, carbon nanotubes have a pronounced tendency to form bunched agglomerates, due to van-der-Waals forces.
Carbon nanotubes are not self-inflammable. Under the influence of an ignition source a mixture of air and carbon nanotubes is explosive (dust explosion). The behaviour in a dust explosion is similar to that of other, carbon-based materials.
Production
Carbon nanotubes can be produced by various methods on the industrial scale. Among these are methods like laser ablation of graphite, the arc discharge between carbon electrodes or chemical vapour deposition (CVD). In CVD hydrocarbons are catalytically decomposed and CNTs can "grow" mainly in parallel on a substrate. In particular it is very important for this method to separate the catalysts from the carbon nanotubes, because otherwise the CNTs cannot be used for all applications mentioned at the start .
Despite many studies, no final judgement can be made on the toxicity of carbon nanotubes, as the observed effects are discussed very controversially in the literature.
Studies on Living Organisms – in vivo
In view of possible adverse health effects as well as with regard to potential medical applications, studies of the distribution of carbon nanotubes inside the body (in vivo) is of great interest .
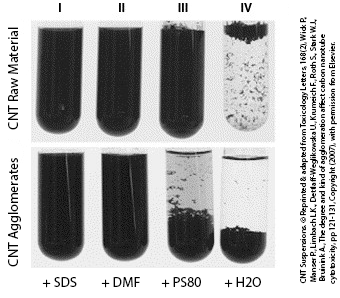
Different suspensions of carbon nanotubes. © Reprinted & adapted from Toxicology Letters, 168(2), Wick P., Manser P., Limbach L.K., Dettlaff-Weglikowska U., Krumeich F., Roth S., Stark W.J., Bruinink A., The degree and kind of agglomeration affect carbon nanotube cytotoxicity, pp 121-131, Copyright (2007), with permission from Elsevier .
Carbon nanotubes (CNTs) are usually delivered into the lungs of laboratory animals by inhalation, instillation or aspiration. No systemic toxicity of multi-walled carbon nanotubes (MWCNTs) could be observed in an inhalation study of several weeks with rats. In case of installation and aspiration, the nanoparticles are first suspended in a liquid and then administered as droplets either directly into the lungs (instillation) or applied dropwise on the tongue followed by an inhalation step (aspiration) .
However, the physiological relevance of both methods is questionable one the one hand because the nanoparticles aggregate within the liquid and on the other hand because the complete dosage is applied at once. A final judgement concerning the risks of exposure to carbon nanotubes under realistic workplace conditions is not possible now due to the limited amount of inhalation studies from independent laboratories.
In different studies, carbon nanotubes were injected either in the abdominal cavity, the veins or into the lungs of laboratory animals. In these cases, CNTs were mostly found in kidneys, spleen and liver or in the lungs. Once within the organs, they are frequently internalised by residential macrophages .
When carbon nanotubes were administered through the stomach, they were excreted within 12 hours without getting into the blood stream. Acute toxicity could NOT be detected in this study regardless of the application method . However, up to now there are only few studies available regarding the influence of carbon nanotubes on internal organs .
Furthermore, carbon nanotubes are a very heterogeneous class of materials. Through various functionalisations, e.g. binding of a fluorescence dye for visualisation in microscopy, the surface properties of CNTs are altered which in turn also changes their behaviour in the body (distribution and retention time) . It is possible to "control" the risk potential of carbon nanotubes by specific surface modification .
But due to the enormous variation of the material (purity, surface properties, applied dose and their agglomeration state, i.e. to which extent single carbon nanotubes stick together and form bigger particles) also the outcome of these studies is differing .
Due to the huge heterogeneity of carbon nanotubes and the differences in test designs and analytics, it remains unclear, how CNTs behave after their uptake into the body. The standardisation of test protocols in the future is absolutely necessary to solve this problem.
Studies Outside the Organism – in vitro

Brightfield image of A549 cells treated with single-walled carbon nanotubes (SWCNTs, highlighted with white arrows). © Karin Pulskamp / Forschungszentrum Karlsruhe, 2009.
As an alternative to in vivo animal tests, the effects of carbon nanotubes (CNTs) are often investigated in cell culture systems (in vitro). Here the CNTs are transferred into a liquid (suspension), then added to the cell culture medium thus bringing the cells into contact with the CNTs (exposure). Despite the plethora of such performed in vitro studies over the last decade, it is not possible to make a final statement on the toxicity of carbon nanotubes due to the differences of the tested CNT materials and applied test methods.
However, some conclusions can be drawn from the published work: bundling (agglomeration) of the carbon nanotubes potentially increases their toxicity . Nevertheless, inhaling of such long or agglomerated CNTs is very limited and due to their large size, they cannot reach the deeper regions of the lung. Furthermore, many of the reported toxic events of carbon nanotubes can be attributed to the high-applied doses, which in reality will not even be achieved with malfunctions during the production.
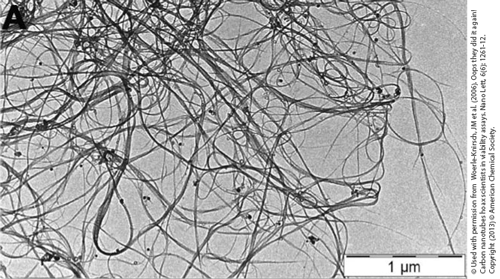
TEM picture of carbon nanotubes (CNTs). © Used with the permission of Woerle-Knirsch, JM et al. (2006). Oops they did it again! Carbon nanotubes hoax scientists in viability assays. Nano Lett, 6(6): 1261-12. Copyright (2013) © American Chemical Society .
Even after production, CNTs are often contaminated with various residues like catalyst metals (in most cases iron, but also nickel, cobalt or molybdenum), amorphous carbon (similar to its appearance in fine dust) or other additives. It has been proven that the observed acute effects were mainly caused by the contaminants .
Carbon nanotubes are considered as very stable and biopersistent. However, experiments demonstrated that an enzymatic degradation of functionalised and non-functionalised single-walled CNTs is possible . Likewise, scavenger cells (macrophages) were able to degrade single-walled carbon nanotubes in vitro .
Using carbon nanotubes in medical applications, e.g. as vehicle for active substances or drugs, requires often some chemical modifications (cross cutting – coatings for nanomaterials). Such modified carbon nanotubes are then e.g. water-soluble and therefore cause no acute toxicity .
In order to deduce reliable conclusions on the toxicity of carbon nanotubes it is necessary to not only thoroughly characterise the materials but also to apply environmentally relevant doses and to use adjusted pharmaceutical dosage forms together with realistic and suitable testing methods .
For the future it is essential to develop suitable models, which on the one hand will analyse the results of comparative in vitro and in vivo studies and which on the other hand will help to measure and characterise the genetic answer (switching-on and –off of genes or genetic programmes) of cells or even the whole organism. With these models in hand, it should be possible to predict the toxicity of a substance or a nanomaterial . Equally, the usage of standardised operating procedures (SOPs) together with internationally accepted reference materials will significantly improve the quality and comparability of in vitro studies.
Carbon nanotubes (CNTs) consist exclusively of carbon, an element present in all living beings and the air. This fact complicates the detection of the CNTs in the environment. There is currently no information on the actual existing amounts of carbon nanotubes in the environment and their distribution in the individual compartments (e.g. water, soil.
An important point for the assessment of potential hazards of nanomaterials for organisms is the determination of actually occurring or expected environmental concentrations. For example, if toxic effects occur only at very high concentrations, which will never occur in the environment, then adverse effects are unlikely, and hence there is no risk for the environment.

© spuno / fotolia.com
Due to the lack of experimental data, researchers simulate the distribution of nanomaterials in the environment with the help of special computer programs in order to predict or to calculate expected environmental concentrations (PEC value). As a starting point for this calculation, the production volumes of carbon nanotubes are used. Since carbon nanotubes are mainly used as composites, only a very small portion passes directly into air or water. The majority is disposed either in landfills or incinerators. Comparing the calculated environmental concentrations with the dangerous levels obtained in laboratory toxicity tests, it appears at present that carbon nanotubes pose no risks to environmental organisms .
Carbon nanotubes are also used for applications such as batteries or textiles. Results of computer simulations for these case products have shown that the CNTs will be released with high probability not only during production, but also during use and disposal . For example, in case of incomplete burning of discarded CNT-containing products low levels of the carbon nanotubes may enter in the environment. When comparing the potentially achievable environmental concentrations of carbon nanotubes with those of other carbon-based materials such as carbon black, then the quantities calculated for the CNTs are considered to be very low .
However, these calculated values need to be confirmed in the future by actual measurements or adapted to increasing production volumes. First methodological approaches for the direct detection of carbon nanotube in the environment have been developed .
Since carbon nanotubes are currently not used in food or cosmetic products, uptake via the lungs, e.g. during production, is the most likely mode of entry into the body. Studies with laboratory animals have shown that carbon nanotubes can penetrate into the deep regions of the lungs, depending on the size.
Uptake via the Lung - Inhalation
In most studies, carbon nanotubes are applied to laboratory animals in an unusual way, making the relevance of these results for our everyday-life questionable. Carbon nanotubes are either instilled, e.g. as a small amount of a high-dosed liquid suspension of nanomaterial, through the airway directly into the pulmonary alveoli (instillation) or applied by sucking-in of the nanoparticle-containing liquid through the throat (aspiration). Both pathways do not correlate to airborne (nano)particles which are present, e.g. at workplaces, and may be inhaled like fine dust.
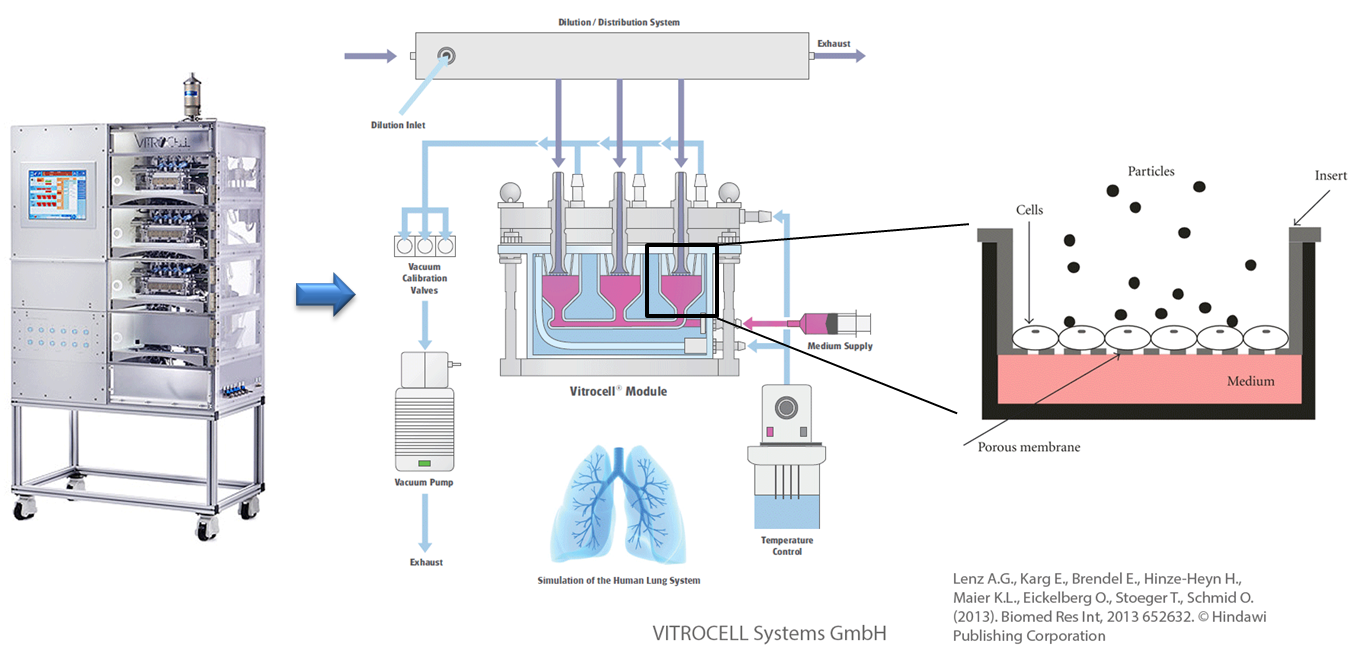
In vitro exposure at the air-liquid interface (ALI) with the example of the Karlsruhe exposure system. Used and adapted with permission from © Vitrocell Systems GmbH, Lenz A.G., Karg E., Brendel E., Hinze-Heyn H., Maier K.L., Eickelberg O., Stoeger T., Schmid O. (2013). Biomed Res Int, 2013 652632. © Hindawi Publishing Corporation
Recent inhalation studies use a more realistic exposure scenario, where carbon nanotubes are exposed to laboratory animals in the breathing air as an aerosol. These studies have shown that carbon nanotubes are able to reach the deeper parts of the lung .

Occupational health & safety measures. © Empa, 2009.
The results of different studies are partly contradictory and therefore discussed critically. Some research groups observed stress reactions of the lung tissue, which were causing inflammation of the lung, formation of granuloma and a change of the lung function. In contrast to that, other studies describe an altered systemic immune reaction (e.g. in the spleen and lymph nodes) without observing any changes in the lung .
These contradictory results can be explained by the different morphologies (modifications) of the applied CNTs, the application method(s) as well as variations within the duration of the studies and the applied doses. Besides these limitations, it is known today that thin, long, biopersistent and fibre-shaped nanomaterials can cause pathogenic effects like inflammation and tumours . For safety reasons carbon nanotubes fulfilling these criteria are no longer in use .
Today there are only few data available on the concentration of carbon nanotubes at industrial workspaces. First laboratory measurements could detect CNTs in the air under certain experimental conditions (ca. 0.7-53 µg/ml3) . Introducing adapted precautionary safety measures, e.g. by the use of laboratory coats and safety equipment such as gloves and respiratory masks, significantly reduces an unintended uptake of nanomaterials.
However, it has yet to be proven especially in case of the CNTs that the critical large and long carbon nanotubes can be taken up via the lung.
Uptake via the Skin - Dermal Uptake

© H.Bauer / fotolia.com
A healthy and intact skin is a very effective mechanic barrier against nanoparticles (cross cutting- nanoparticles & skin), which was also shown for titanium dioxide particles by the European project NanoDerm . To date, no transfer of nanoparticles into blood vessels across the skin could be observed and whether carbon nanotubes (CNTs) can be taken up across the skin has not been investigated until now.
Nevertheless, a conclusion using an analogy might be helpful in this case:
Tattooing ink, especially black ink, contains carbon particles and is mechanically deposited via a tattoo needle in the deeper layers of the skin. From there, only very few of these particles are transported into the nearby lymphatic vessels while most of them remain in place explaining the long lifetime of tattoos. Since the surface characteristics of carbon nanotubes are very similar to those of these carbon particles, it can be inferred that transport of the CNTs via and within the skin will be very weak.
Uptake via the Gastro-Intestinal Tract
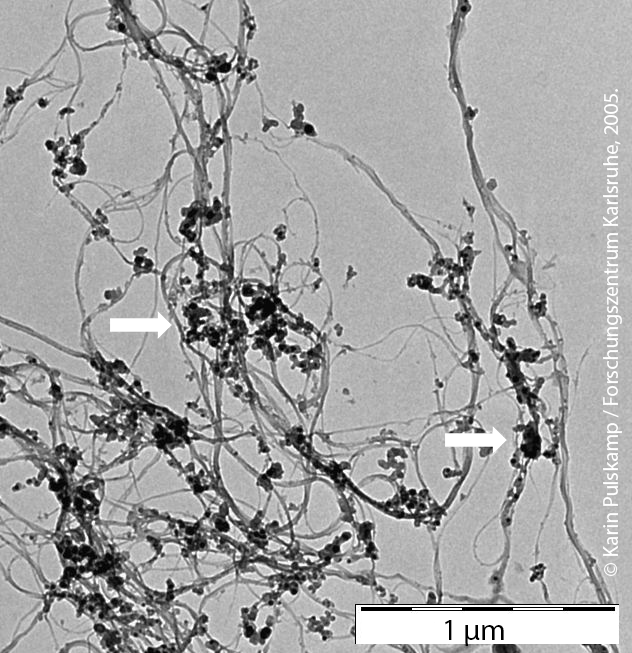
Single-walled carbon nanotubes (SWCNT) with carbon impurities (spherical deposits highlighted with white arrows) © Karin Pulskamp / FZK, 2005.
Carbon nanotubes (CNTs) are not accredited for use in foods, and there are no indications for this to happen in the near future. However, it is theoretically possible that e.g. inhaled carbon nanotubes are able to reach the gastro-intestinal tract through coughing followed by swallowing and further distribution within the body. So far, only few analyses exist on this topic.
Water-soluble carbon nanotubes, which were directly administered into the stomach of mice CNTs, stayed only for a short period in the gastro-intestinal tract before being excreted with the faeces within 12 hours. The carbon nanotubes did neither enter the bloodstream nor cause any biological effects . In case of short or long multi-walled carbon nanotubes (MWCNTs), which were administered directly to the stomach of mice, only long and stiff fibre-shaped CNTs caused inflammation and damages of the upper cell layer. These observed effects (pathogenic reactions) have been known for many years in particular for long and stiff asbestos fibres .
However, if unmodified carbon nanotubes are injected directly into the bloodstream of laboratory animals, these CNTs are distributed over different internal organs (e.g. liver, spleen, lung) and are excreted only to a small amount via the urine and faeces .
Similar to the uptake of carbon nanotubes via the lung, it is not possible to make a final statement for CNTs in the gastro-intestinal tract. The behaviour of carbon nanotubes strongly depends on the respective modification, the way of administration and their dosage.
The term "carbon nanotubes" (CNTs) comprises a group of carbon-containing nanomaterials with diverse properties, which for example differ in their surface structure or in the number of walls. These features significantly affect the strength of the toxic effects of the CNTs. Many of the observed effects are due to the fibre-like form of the carbon nanotubes, which may cause indirect effects upon contact with the surface of the environmental organisms. Universally valid statements about the environmental toxicity of carbon nanotubes are not yet possible .
Due to their fibre-like form carbon nanotubes cause indirect toxic effects in bacteria by acting as needles which pierce the cell envelope leading to mechanical damage. Via those defects in the sheath the growth of bacteria is severely restricted or totally inhibited. This effect on bacteria gets stronger the better the CNTs are distributed and isolated in the nutrient solution .
Single-celled organisms such as ciliates and amoebae, which are present in all water bodies, are able to take up CNTs but equally able to excrete those as well. Their viability is not affected by the carbon nanotubes, however, food intake (bacteria) and digestion is inhibited .
Various types of algae are also indirectly affected by carbon nanotubes. Pure and coated CNTs inhibit the growth of algae by shading the sunlight or by forming agglomerates between algae and CNTs . If vital light is missing, the algae cells can produce less energy and consequently, growth is inhibited. Other factors such as the nature of the surface of the CNTs or the agglomeration state in the aqueous environment have little effect on algal growth . In addition, the carbon nanotubes can also induce oxidative stress in algae .
 When adding carbon nanotubes to the soil of various crops such as wheat or rape, the plant roots take up only very small amounts (0.005 %) of the offered CNTs . Hence an accumulation of CNTs in the food chain via crop is considered unlikely. The growth of the plants was not affected. In tomato plants, incorporated carbon nanotubes could be detected in roots, shoots and fruits. Also carbon nanotubes were found to activate stress genes in the plant cells, indicating an increased stress to the plant . Perhaps the plant is actively trying to excrete the CNTs. But it is not yet clear how the carbon nanotubes induce stress in the plant cells.
When adding carbon nanotubes to the soil of various crops such as wheat or rape, the plant roots take up only very small amounts (0.005 %) of the offered CNTs . Hence an accumulation of CNTs in the food chain via crop is considered unlikely. The growth of the plants was not affected. In tomato plants, incorporated carbon nanotubes could be detected in roots, shoots and fruits. Also carbon nanotubes were found to activate stress genes in the plant cells, indicating an increased stress to the plant . Perhaps the plant is actively trying to excrete the CNTs. But it is not yet clear how the carbon nanotubes induce stress in the plant cells.
 Water fleas take up carbon nanotubes regardless of their surface charge and excrete them likewise . Generally, the surface charge contributes significantly to the behaviour of the CNTs in aqueous media (stability, agglomeration) and can influence various parameters such as uptake and distribution. In this study, however, the differences observed in the strength of the toxic effects could not be attributed to the uptake, but rather to the coating of the carbon nanotubes .
Water fleas take up carbon nanotubes regardless of their surface charge and excrete them likewise . Generally, the surface charge contributes significantly to the behaviour of the CNTs in aqueous media (stability, agglomeration) and can influence various parameters such as uptake and distribution. In this study, however, the differences observed in the strength of the toxic effects could not be attributed to the uptake, but rather to the coating of the carbon nanotubes .
The effects of carbon nanotubes with various surface charges were also studied in earthworms . As well as in water, the behaviour of the CNTs in soil varies depending on the particular surface properties. Similar to water fleas the differences in carbon nanotube behaviour in soil had no influence on absorption and excretion in earthworms. There are currently no results regarding toxic effects in worms available.

Larvae of the African clawed frog show an acute toxic reaction towards carbon nanotubes. The CNTs "clog" the gills and intestine thereby severely restricting the growth and thus the survival of the larvae . No genotoxic effects were observed.
 Single-walled carbon nanotubes added to the food of the rainbow trout are incorporated by the fish, but they do not exert harmful effects . If the carbon nanotubes however are added directly to the water, then the trout shows an irritation of the gills accompanied by an accelerated respiration . Similar to situation with the frog larvae, the CNTs clog the gills of the fish due to their fibrous form and cause the mentioned observed effects.
Single-walled carbon nanotubes added to the food of the rainbow trout are incorporated by the fish, but they do not exert harmful effects . If the carbon nanotubes however are added directly to the water, then the trout shows an irritation of the gills accompanied by an accelerated respiration . Similar to situation with the frog larvae, the CNTs clog the gills of the fish due to their fibrous form and cause the mentioned observed effects.
Functionalized multi-walled carbon nanotubes that were directly injected into zebrafish embryos are no longer detectable in the animals after 4 days, and were, therefore, effectively eliminated . The further development of fish embryos was not affected. However the next generation showed significantly reduced survival rates.
When looking at the entire life cycle of carbon nanotubes - from production and use to disposal - then the manufacturing of the CNTs has a much greater impact on environmental organisms than the direct release of the CNTs in the environment . For example, indirect effects to the environmental organisms can be caused by environmental damage that may occur due to mining of raw materials or due to exhaust gases created.
Finally, the toxicity of carbon nanotubes is currently difficult to assess due to their diversity. Mechanical and indirect effects due to the fibre-like form are observed in many organisms, particularly in studies in which very high doses were tested. For very low concentrations, as they are currently expected in the environment, no risk to environmental organisms is currently estimated.
Carbon nanotubes are absorbed by the body depending on the mode of administration and type (single-walled, multi-walled, modified) and can be found in various organs and cell types.
Behaviour inside the body
Carbon nanotubes show a typical "fibre-like" behaviour with the length of the individual CNT fibres playing a decisive role for the severity of their biological effects. Based on currents studies, the uptake of CNTs across the lung is the most critical entry pathway for carbon nanotubes into the body, because fibres have a longer residence time in the lung compared to spherical materials. Therefore, the aspect ratio of diameter to length is the determining factor for CNT-fibres .
Carbon nanotubes of a certain length, like other fibre-shaped harmful materials, cannot be removed from the lung by regular cleaning processes. The fibres keep being stuck in the lung tissue causing a permanent irritation of the tissue. This may even go so far that the fibres migrate into deep tissue layers causing an inflammation for years or decades, which ultimately may trigger tumour development . This is in perfect agreement with a recent study in rats which demonstrated exactly this relationship between the length of the carbon nanotubes and their effect on the formation of granuloma within the treated animals .
These effects also explain the comparison with asbestos fibres, although this is only applicable to very long fibres (>5-10 µm). Short carbon nanotubes do not show this effect and can therefore be used in technical applications .
Behaviour at the Blood-Brain Barrier
Preparation of injectable medicines. © sudok1 / fotolia.com
Up to now, only one single study described the detection of very small amounts of unmodified, water-insoluble carbon-nanotubes in the brain of laboratory animals after CNT injection into the bloodstream. This observation indicates the ability of carbon nanotubes to cross the blood-brain barrier under certain conditions .
However, to date no validation of this distribution-behaviour has been published. Furthermore, the study applied relatively high doses of CNTs and did not describe in detail the analysis of the brain-tissue. It is quite conceivable that small bundles of CNTs were located within the brain-capillaries and therefore assigned to the brain without ever having left the vessels.
It remains to be seen whether other studies can confirm this behaviour and under which conditions a transfer of carbon nanotubes from the bloodstream into the brain is possible. Once identified, this mode of action could have positive (e.g. drug administration in the brain) as well as negative effects (e.g. cell death, brain damage), which still needs to be studied in more detail.
Behaviour of Uptake in somatic cells
![Paradigm of frustrated phagocytosis for asbestos fibres and carbon nanotubes. © Used & adapted with permission from Donaldson, K et al. (2010). Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol, 7 5. Open Access article [CC-BY-2.0 (http://creativecommons.org/licenses/by/2.0)]. Paradigm of frustrated phagocytosis for asbestos fibres and carbon nanotubes.](https://nanopartikel.info/wp-content/uploads/2020/11/Donaldson-2010_EN-web.png)
Paradigm of frustrated phagocytosis for asbestos fibres and carbon nanotubes. © Used & adapted with permission from Donaldson, K et al. (2010). Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol, 7 5. Open Access article [CC-BY-2.0 (http://creativecommons.org/licenses/by/2.0)] .
Without any stabilising additives, nanomaterials and also carbon nanotubes (CNTs) tend to agglutinate very rapidly. Since such agglomerates can be larger than a normal cell, the cells would rather "choke to death" on the carbon nanotubes (frustrated phagocytosis) than take them up.
Smaller agglomerates are taken up by the cells via pinocytosis and can be detected inside the cells by means of electron microscopy (TEM) in cell inclusions (so-called vesicles). The vesicular membrane protects the remaining cell components from the carbon nanoparticles, which means that the nanoparticles are inside the cells but still encapsulated. However, one study showed the possibility of CNTs to escape these encapsulations and to reach the cytoplasm of the cells .
Modified carbon nanotubes, which can overcome normally insurmountable biological barriers due to modified surface properties, are of great interest to medical and biotechnological applications. In contrast to non-modified CNTs, the functionalised carbon nanotubes do not agglomerate in aqueous environments and are therefore available as individual tubes . Due to their shapes and sizes, these CNTs can enter cells via an alternative, vesicle-independent path. Recent studies have proven that specific contents of lung surfactant (proteins and lipids) produce such a "biological functionalisation" of multi-walled carbon nanotubes, which does not affect the cytotoxicity of multi-walled CNTs .
So far, it has not yet been adequately explained in which way the CNTs can leave the cells afterwards and whether they accumulate inside the cells.
Although they consist only of a carbon skeleton, carbon nanotubes (CNTs) have a plurality of different structures: single-walled and multi-walled tubes, different lengths, and surface coatings. These variations make it difficult to directly compare the environmental behaviour of carbon nanotubes. They also are often not present as single tube, but are aggregated to bundles. Therefore it is difficult to generalise about the environmental fate of this great diversity of CNTs.
![Overview on different carbon nanotube structures: single-walled (SWCNT), double-walled (DWCNT), multi-walled (MWCNT) and possible surface modifications. © Adapted with permission from Jackson, P et al. (2013). Bioaccumulation and ecotoxicity of carbon nanotubes. Chem Cent J, 7(1): 154. Open Access article [CC-BY-2.0 (http://creativecommons.org/licenses/by/2.0)]. Overview on different carbon nanotube structures: single-walled (SWCNT), double-walled (DWCNT), multi-walled (MWCNT) and possible surface modifications.](/wp-content/uploads/2020/11/CNT-Jackson-2013-EN.png)
Overview on different carbon nanotube structures: single-walled (SWCNT), double-walled (DWCNT), multi-walled (MWCNT) and possible surface modifications. © Adapted with permission from Jackson, P et al. (2013). Bioaccumulation and ecotoxicity of carbon nanotubes. Chem Cent J, 7(1): 154. Open Access article [CC-BY-2.0 (http://creativecommons.org/licenses/by/2.0)] .
The environmental behaviour of carbon nanotube strongly depends on the shape and properties of the tubes. They may e.g. be water soluble or insoluble in water, which essentially affects their behaviour in surface waters.
Uncoated carbon nanotubes hardly dissolve in water and therefore deposit quickly (sediment). An improved water solubility and hence a longer residence time in water can be achieved by modification of the CNT surface, for example by various coatings . Dissolved organic substances (e.g. decomposition products of dead organisms) stabilise multi-walled carbon nanotubes in surface waters to prevent sedimentation to the bottom of the water . An opposite effect is caused by calcium ions. They lead to an agglomeration of carbon nanotubes into larger bundles resulting in an accelerated sedimentation .
Even in soil the surface properties of the CNTs crucially influence their fate. For example, single-walled carbon nanotubes have a low mobility if their solubility in water has been improved by a specific coating. Due to their fibre-like form the CNTs quickly settle and hence have limited transport upon release .
Similar to other well-known carbon-based materials (e.g. activated carbon), carbon nanotubes can be very effective in binding other substances and are thus applied as filters. Based on this property, carbon nanotubes can also bind and accumulate numerous environmental pollutants . Influence on the pollutant binding has the pollutant itself , but also the available free surface area and the extent of surface modification of the CNTs . Likewise, binding of the contaminants to the carbon nanotubes can be influenced by the presence of copper ions or surfactant . Copper ions increase the pollutant binding by connecting the CNTs with the pollutant like a "bridge". In contrast, the components of the surfactant occupy the surface of the carbon nanotubes, so that hardly any space for pollutants remains. Compared to carbon black, however, the environmental concentrations of the CNTs are considered to be very low, hence the pollutant binding to CNTs is expected to be minimal .
There is preliminary evidence that carbon nanotubes can be degraded by certain plant enzymes . Unmodified CNTs are protected from these enzymes whilst carbon nanotubes with certain surface modifications (e.g. carboxyl groups) can be well degraded .
In summary, the behaviour of carbon nanotubes in the environment is dependent on their properties, such as length and surface state. In addition, the CNTs interact in the environment with numerous other substances. The differences in behaviour also influence the type or degree of toxicity towards environmental organisms.
 >
>