
ceramic knives © Unclesam / Fotolia.com
Zirconium dioxide or zirconia is one of the most widely used ceramic oxides. Its applications range from use in abrasive products, dental bridges and crowns, additive in paints and lacquers, in fuel cell membranes and in joint implants. Zirconium dioxide is also utilised as white pigment for porcelain or in a mixture with vanadium oxide as yellow pigment. Most consumers associate the material with ceramic kitchen knives found in many households nowadays and their blades are made of zirconia.
Most products on the market are manufactured based on micro-crystalline zirconium dioxide powders, only niche markets have started to increasingly use powers of zirconium dioxide nanoparticles.
How can I come into contact with this material?
In general, there is very little chance to come into contact with zirconium dioxide particles since these are firmly embedded in plastics or other materials. Looking at medical applications the picture changes completely. Dental prosthesis and joint implants are made of zirconia ceramics and in addition, zirconium dioxide is added as contrast agent to bone cements for radiology purposes during surgical procedures.
Is there any risk from this material to humans and the environment?
Zirconium dioxide has been proven to have excellent compatibility with bones and the surrounding connective tissue. Likewise zirconium dioxide nanoparticles are non-toxic to other environmental organisms such as bacteria, algae and zebra fish.
Conclusion
There is little chance of coming into contact with free zirconium dioxide nanoparticles particles as only very few applications make use of this material in nanoscaled form. Zirconium dioxide ceramics were recognised a long time ago to be highly suitable for bone implants. Many studies have been performed and confirm an excellent compatibility of zirconium dioxide with human tissues.
By the way…
Zirconia crystals (so-called cubic zirconia) are used as diamond imitations for jewellery since zirconium dioxide has, similar to real diamonds, a high refractive index.
Properties and Applications
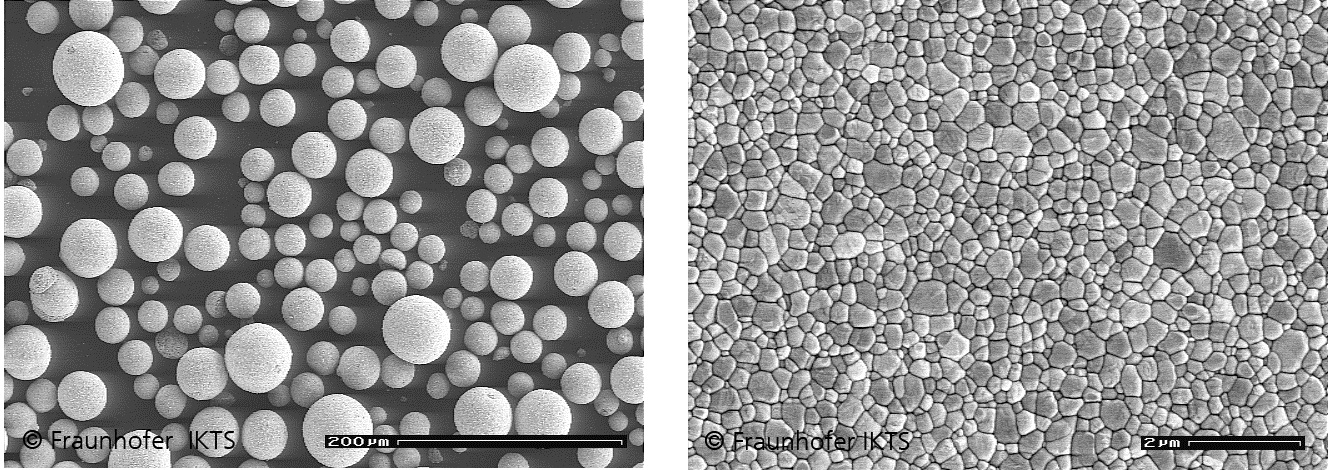
TEM image of commercial zirconium dioxide granules (right). After pressing and sintern of the pellets the zirkonia cermaic is produced (left)© Fh IKTS
Zirconium dioxide (ZrO2), which is also referred to as zirconium oxide or zirconia, is an inorganic metal oxide that is mainly used in ceramic materials. Zirconium dioxide succeeds zirconium as the compound of the element zirconium that most frequently occurs in nature. It is a heavy metal of which 0,016 % is found in the earth crust and which, thus, occurs more frequently than the elements chlorine and copper. Its great hardness, low reactivity, and high melting point have made it the oldest mineral that can be found on the earth. Zirconium does not occur massively but is bound in minerals, mainly in zircon (ZrSiO4).
Zircon is also known as a precious stone whose color may vary from colorless white to brown, green, etc., depending on the traces of impurities. Due to their high optical density, zircon (and zirconia) gems have high refraction indices. Provided they are pure and large enough, they are suited, therefore, as (cheaper) substitutes for diamonds. None of the natural isotopes of zircon is radioactive. Yet, since zircon is relatively often impurified with uranium oxides and other radioactive substances such as thorium salts, it is responsible for much of the natural radioactive radiation. Geological age determination through radioactive dating, for example, makes use of such impurities.
Zirconium dioxide is the most important zirconium compound which due to its properties is used in various products. In nature, ZrO2 occurs in the mineral form as baddeleyite, a modification in monoclinic crystal lattices (which is often found as weathered grit in gravel). Zirconium dioxide is non-magnetic and highly resistant against acids, alkaline lyes, and exogenous (chemical, thermal, and mechanical) influences.
Zirconium dioxide has a high thermal stability. It does not melt below 2680°C, which is why it is used in high-temperature ceramics such as crucibles or furnaces. Since, in addition, it has a high mechanical stability and is very resistant to abrasion, it serves to e.g., improve the properties (especially the scratch resistance) of varnishes and coatings applied as top coats to automobiles, or as finishes to parquets and furniture. Zirconium dioxide is also found in varnishes for electronic items, in nail polishes, in ink jet printer’s inks, and other products. Besides, it is known as an abrasive and is found (like titanium dioxide) as a white pigment in porcelain.
Moreover, hip joint endoprostheses and other high-performance medical ceramics benefit from the advantages of zirconium dioxide. Dentistry makes use of its special properties when manufacturing corona frames and bridge frames, tooth root studs, and metal-free dental implants. Zirconium dioxide is the most widely used oxide ceramic next to aluminium oxide. Thanks to its electrolytic conductivity, it was used as early as in 1897 in the incandescent bodies (ceramic rods) of the Nernst lamp, an electrically powered incandescent lamp invented by the German physicist and chemist Walther Nernst.
Zirconium dioxide is not self-inflammable as nanometer-sized powder. Also as a mixture with air (dust) under the influence of an ignition source, it is not inflammable, so there is no possibility of a dust explosion.
NanoCare Data Sheets
- Zirconium Dioxide data sheet No.1
- Zirconium Dioxide data sheet No.2
- Zirconium Dioxide data sheet No.3
Further Information
- IFAGESTIS-database on hazardous substances (en):Zirconium(IV) oxide(last access date: Feb 2011).
- Breuer, H (2006). dtv-Atlas Chemie - Grundlagen und Ergebnisse der modernen Chemie, Band 1, 10. Auflage, dtv-Verlag, München. ISBN 978-3-423-03217-9. (in GERMAN)
- Roempp Chemielexikon (1992). 9. Auflage, Falbe, F & Regnitz, M. Georg Thieme Verlag Stuttgart, New York. (in GERMAN)
- Katz, F (2007). Literaturübersicht über Zirkoniumdioxid in der Zahnmedizin und Bruchbelastbarkeit am Beispiel von Slot-Inlay Brückengerüsten, Inaugural-Dissertation, University of Freiburg, Germany. (in GERMAN)
Studies showed that these particles only cause negative effects in very high doses.
General Hazards
An investigation of workers exposed with zirconium dioxide did not result in differences compared to control groups. A small group of workers induced an investigation in 1981. They had been exposed to zirconium at the work place for 1 to 17 years and suspected a possible health risk. The subsequent study within this collective of 32 men resulted in no differences to a corresponding control group . Nevertheless, the size of the collective is too small to come to final estimations of a possible health effect.
Studies on Living Organisms – in vivo
In animal testing the zirconium-treated animals (zirconium salts in drinking water) survived significantly longer than the non-treated control animals . Rats exposed to 10g/kg ZrO2 showed no negative symptoms. The animals have been treated over the whole lifetime with soluble metal salts in the drinking water. Zirconium did accumulate in several organs (e.g. spleen and heart), but this induced no negative effects especially on the overall lifetime which was prolonged in the zirconium-treated rats compared to the control animals.
Studies Outside of Organisms – in vitro
Within the project NanoCare three different modifications of zirconium dioxide were used. Furthermore a threshold concentration of at least 50 µg ZrO2 particles per cm2 cell culture area was determined by means of in vitro tests on the human lung epithelial cell line A549. At this concentration as the lowest one that triggers an effect, cells were found to become stressed. Investigations with 11 different cell lines from different derivations and up to 10 µg particles per cm2 cell culture area used, none oft the cell lines showed indications of stress or zirconium dioxide caused other cell-damaging effects. Likewise, a test on apoptosis was negative with cell cultures.
Within co-culture systems which allow to better represent the in vivo situation in the body due to simulate the interaction of the cells, particles also showed no adverse effects . The treatment of macrophages with ZrO2-particles (600 nm in diameter) at concentrations up to 2.500 particles per cell induced no observable effects .
There are no data regarding environmental exposure with zirconium dioxide nanoparticles available.
No valid studies are currently available on absorption via the lungs, skin or gastrointestinal tract.
No toxicity of nanoscale zirconium oxide was observed in bacteria and algae . Likewise, no toxic effects were found in the zebrafish embryo .
Zirconium dioxide is used as bone implants. It is said to have excellent compatibility with the bone tissue and the surrounding connective tissue.
Zirconium dioxide has been investigated n many studies for its possible use in implants. Here we refer to one example which demonstrated “an excellent compatibility with autogenous bone and the surrounding soft tissue” . The period of treatment was up to one year. To test for further effects another study investigated the biological effect of aluminium hydroxide or zirconium carbonate which has been injected into the skin. This study revealed that aluminium hydroxide induced the formation of granuloma within the skin, but ZrCO3 had none of such negative effects .
Another important investigation dealt with the interaction of nanoparticles with specific peptides and proteins and if such a binding can alter the behaviour of the proteins . The reaction measured was the aggregation of small proteins within the nervous system which play a role in neurodegeneration diseases as Alzheimer or Parkinson disease. Zirconium dioxide exibits no interaction with peptides and proteins. Of course the experimental set-up was very artificial which means that these particles have not been shown to reach the brain in a living organism and induce exactly such a behaviour of the proteins but it may present a first insight into the possible reactions.
The experiments shown by the Chinese group didn’t reveal such a negative effect. As early as 1993 it was demonstrated that particles have been produced by abrasion from ceramic surfaces of non-fitting implants which are often nanosized . This medical investigation found an influence on the surrounding tissue by these “ultrafine particles” and traced this back to the smallness of the particles and not to the material they consisted of. Slightly larger particles around 1 micrometre have been investigated for their influence on human stem cells. Concentrations as high as 500 to 5000 particles per cell had a slight effect on the viability of these cells . Compared to particles made from other materials zirconium dioxide had always the less pronounced effects on stem cells even they had one.
To date, no data regarding the environmental behaviour of zirconium dioxide nanoparticles are available.
 >
>