 >
Spotlight Juli 2020: “Nanosafety – More than just regulatory processes”
>
Spotlight Juli 2020: “Nanosafety – More than just regulatory processes”
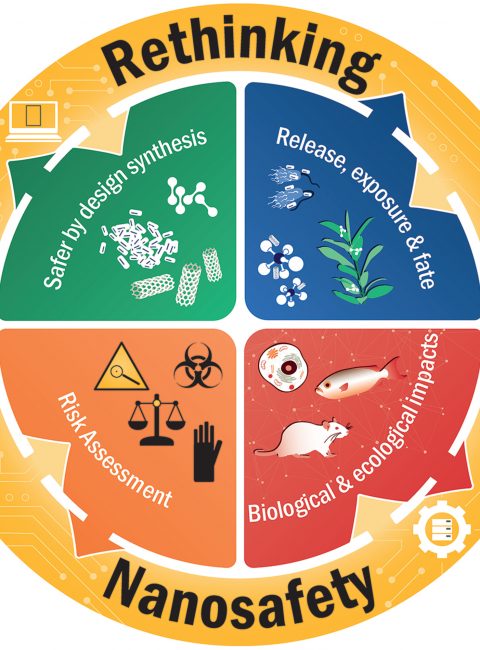
Nanosafety is more than just a compulsory aspect of nanomaterials research and regulation. This research area also has great potential to drive new innovations. It is exactly this perspective that is addressed in the special issue “Rethinking Nanosafety: Harnessing Progress and Driving Innovation” by Chen et al. 2020.
The article illustrates that especially in the field of biology and nanomedicine, safety assessment can contribute to a more fundamental mechanistic understanding of the interaction between nanomaterials and living systems. Innovations based on this can range from personalised nanomedicine to tailor-made nanomaterials for the agricultural industry. In addition, the development of advanced cell and tissue models could further advance the understanding of so-called “adverse outcome pathways” (AOPs) as well as their development and validation.
The Special issue Rethinking Nanosafety brings together more than 50 research projects of renowned scientists in the field of nanosafety and reflects the cooperative and future-oriented character of this research area. The first part of this special issue was published in Small in May 2020. The publication of the second part will follow in July 2020.
Original Publication:
Chen, C., Leong, D.T. and Lynch, I. (2020), Rethinking Nanosafety: Harnessing Progress and Driving Innovation. Small, 16: 2002503. DOI:10.1002/smll.202002503
Weitere Spotlights
Spotlight June 2023: New catalytic process for recovering important materials from composites in a single process
Previously virtually impossible and a huge problem: fibre-reinforced resin composites (epoxides) were not recyclable, and wind turbine rotor blades, for example, add up to a waste pile of 43 million tons by 2050. Researchers have now taken an important first step in “reprocessing” these composites and catalytically dissolving them so that the carbon fibres and […]
Read moreSpotlight May 2022: Nano-ghosts” – Risk assessment of submicron-sized particles in food biased towards fictional “nano”
The European Commission has issued a ban on the colorant titanium dioxide in food. Titanium dioxide, which provides a nice shine and bright white color, can potentially damage genetic material. We chose a review article from 2022 for the May 2022 Spotlight that addresses the risk assessment of food-grade titanium dioxide (E171) and the resulting […]
Read moreSpotlight January 2021: Nanoplastics challenge – How to improve tracking of nanopolystyrene distribution in the environment.
In January, we present a paper published in the Nature Journal communications materials. The article focuses on the development of a new detection method of nanopolystyrene. The method not only makes it possible to detect nanoplastics in the environment for the first time, but also to determine their accumulation in plants and animals. Nanoplastics, which […]
Read moreSpotlight December 2021: Silica nanoparticles improve plant disease resistance
The resistance of plants to various pathogens is often increased in agriculture with various chemicals (“fertilizers”). A new direction is being taken with the use of nanoparticles. These can be sprayed on the plants. In the present study, the model plant Arabidopsis was used to investigate whether silicon dioxide nanoparticles (SiO2) can increase resistance to […]
Read more


