Engineered nanomaterials can be released from nanoproducts during use and waste disposal and a fraction can end up in wastewater. The function of wastewater treatment plants is to purify polluted water which includes the removal of nanomaterials. However, they can potentially negatively affect the purification potential of wastewater treatment plants
How do nanomaterials get into wastewater?
Nanomaterials are contained in many different products and can be released during their whole life cycle, i.e. via production, use and disposal (see crosscutting article on Nanomaterials in waste) which finally can end up in wastewater. The type of product as well as the type of incorporation of the nanomaterials (e.g. bound or free) influence greatly the released amounts of nanomaterials. Nanomaterials from cosmetics for example end up to a large extent in wastewater while nanomaterials embedded in a polymer matrix, e.g. in a bicycle frame, are hardly released. With the currently available analysis methods it is very difficult to measure the amounts of engineered nanomaterials present in wastewater under real conditions (see crosscutting article on Detection of nanomaterials in the environment). Therefore computer-based simulation models are used to estimate the amounts of released nanomaterials present in wastewater. These models simulate the life cycle of the nanoproducts and calculate the expected nanomaterial flows into wastewater (see Estimating the occurrence of nanomaterials in the environment ). The newest models show that large amounts of nanoparticles, mostly titanium dioxide and zinc oxide, are discharged into wastewater treatment plants [1].
What happens to nanomaterials in wastewater treatment plants?
During wastewater treatment particles and compounds affecting the environment are removed from wastewater. Generally, a wastewater treatment plants comprises a mechanical stage to separate solids, a biological stage to degrade organic materials and further stages for nitrogen- and phosphorous removal. At the end of wastewater treatment, purified wastewater is discharged into natural waters while the removed solids are contained in the separated sewage sludge.
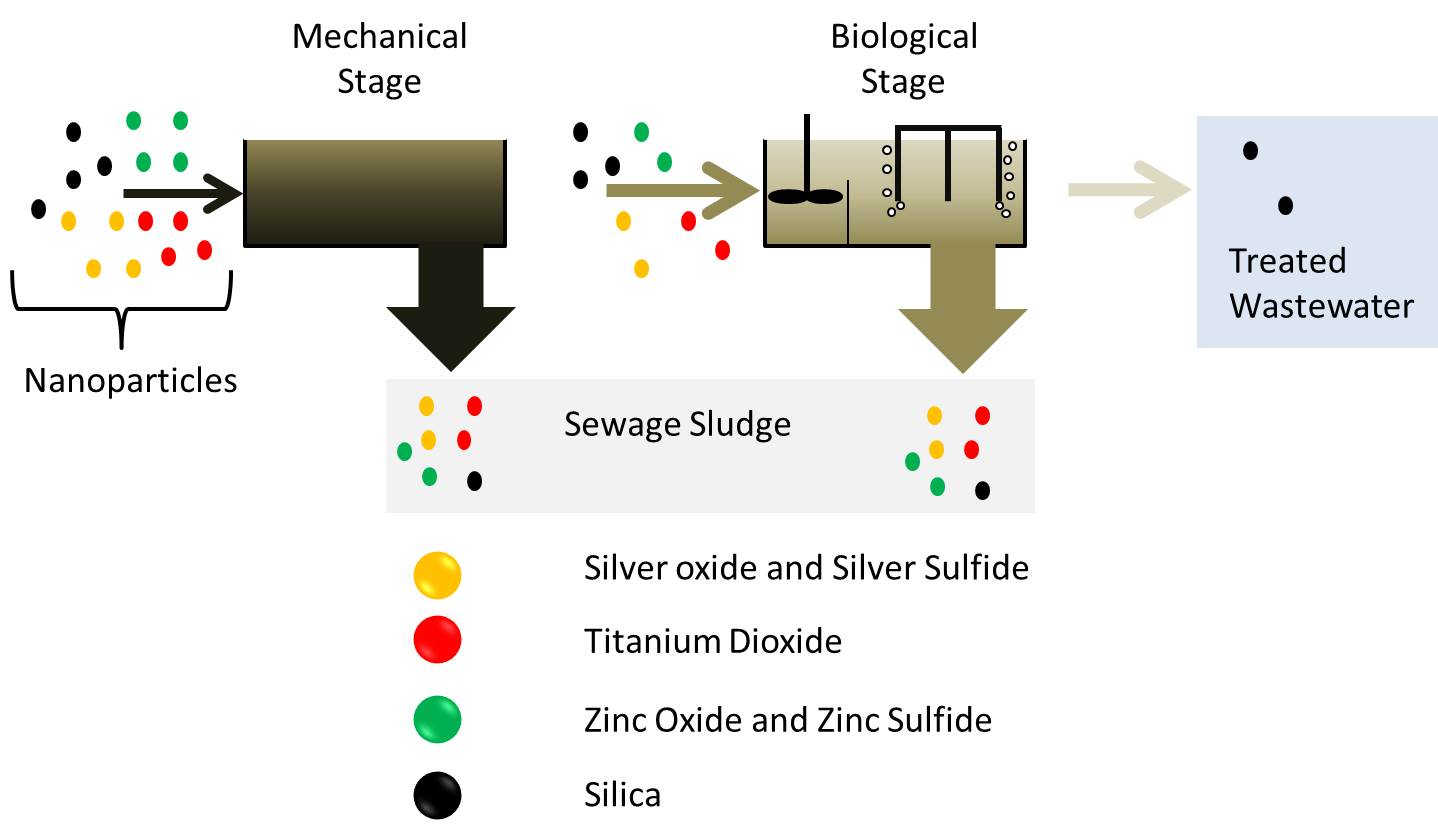
Removal of nanoparticles during the different stages of wastewater treatment plants © UFZ
Based on the current knowledge, most of the investigated nanoparticles like nano silver, titanium dioxide or zinc oxide are removed by 90 to 95% from wastewater [2]. An exception are silica nanoparticles which are less efficiently removed due to their peculiar surface properties and which may therefore be present under certain conditions in treated wastewater [2]. The removed nanoparticles become part of the sewage sludge which is separately treated. Despite the very high removal rate of nanomaterials during wastewater treatment, computer-based simulation models show that treated wastewater is one of the main sources of nanomaterials to freshwaters [1]. In addition, we have to consider that untreated sewage can get into the environment. Reasons for this can be sewers that are not connected to a wastewater treatment plants or strong rain events. During such rain events, a part of the wastewater is discharged untreated into the environment to avoid an overload of the wastewater treatment plants. Measurements in rivers have shown that the discharge of untreated wastewater can indeed result in a temporary increase in the concentration of nanomaterials in the water [3].
Nanomaterials in sewage sludge

Sludge treatment in thickeners in the sewage treatment plant photographed from above © Werner – stock.adobe.com
The solids separated during wastewater treatment are collected as sewage sludge. This sludge consists mainly of biota, organic and inorganic compounds. Nanomaterials made of metals or metal-oxides such as silver or titanium dioxide are not biologically degradable and are enriched in sewage sludge [5,6]. In addition to the removal from wastewater, some of the often-used nanomaterials such as silver, zinc oxide and copper can undergo transformation reactions and form new minerals, for example with sulfur [4-8]. These nanomaterials are therefore not degraded but they lose by this transformation their original properties. In case of a combination with sulfur, the formed metal-sulfides are quite insoluble under environmental conditions and are much less toxic to environmental organisms than the original nanoparticles [9]. The further fate and the detailed ecotoxicological effects of the transformation products are part of ongoing investigations.
There are several options how sewage sludge can be disposed or further used. In accordance with country-specific regulations, sewage sludge is incinerated, landfilled or used as fertilizer in agriculture. In Germany the largest part of sewage sludge is currently incinerated, only a small fraction is used in agriculture or landscaping. During incineration of sewage sludge the nanomaterials remain in the ashes and are finally landfilled, while they are completely transferred to soils and therefore to the environment in the case of agricultural use. Computer-based simulation models have shown that sewage-sludge treated soils can reach over the years very high nanomaterial concentrations of several mg/kg [1]. Monitoring studies could confirm this prediction for titanium dioxide, silver and zinc oxide nanomaterials [10-12]. However, despite the increased burden of soils by long-lived metal compounds, only a small fraction of the metals present in sludge-treated soils can actually be attributed to nanomaterials [13, 14].
Influence of nanomaterials on the purification capacity of wastewater treatment plants
Nanomaterials can potentially negatively influence the purification capacity of wastewater treatment plants. They could for example affect the biological stage of wastewater treatment, e.g. the degradation of organic matter or the elimination of nitrogen and phosphorous [15]. In particular silver and zinc oxide nanoparticles are well known for their inhibitory effect on bacteria. However, these effects only occur at nanoparticle concentrations that are higher than those occurring in real wastewaters. Because the actually present concentrations of nanomaterials in wastewater are low, it is currently assumed that nanomaterials do not affect the biological stage of wastewater treatment plants under realistic conditions [15].
In conclusion we can state that a large fraction of the nanomaterials is removed very efficiently from wastewater during wastewater treatment and is enriched in the sewage sludge. Given the low concentrations of nanomaterials expected in wastewater, the purification capacity of wastewater treatment plants should not be affected negatively by nanomaterials. However, the potential of an enrichment of nanomaterials in soils exists if sewage sludge is used as a fertilizer in agriculture.
Literature
- Sun, TY et al. (2014) Environ. Pollut., 185 69-76.
- Wu, J et al. (2017). Water, Air, & Soil Pollution., 229(1): 9.
- Wang, S et al. (2017). RSC Advances, 7(59): 37065-37075.
- Loosli, F et al. (2019). Environmental Science: Nano, 6 763-777.
- Polesel, F et al. (2018). (2019). Water Research, 141 19-31.
- Westerhoff, P et al. (2011). Journal of Environmental Monitoring, 13(5): 1195-1203.
- Kaegi, R et al. (2011). Environmental Science & Technology, 45(9): 3902-3908.
- Lombi, E et al. (2013). Environmental Pollution, 176 193-197.
- Lombi, E et al. (2012). Environmental Science & Technology, 46 9089−9096.
- Ma, R et al. (2014). Environmental Science & Technology, 48(1): 104-112.
- Gogos, A et al. (2017). Environmental Science: Nano, 4(8): 1733-1741.
- Levard, C et al. (2013). Environmental Science & Technology, 47(23): 13440-13448.
- Yang, Y et al. (2014). Science of the Total Environment, 485 441-449.
- Brunetti, G et al. (2015). Water Research, 77 72-84.
- Donner, E et al. (2015). Environmental Pollution, 205 78-86.
 >
>