 >
Spotlight February 2022: Probabilistic risk assessment – the keystone for the future of toxicology
>
Spotlight February 2022: Probabilistic risk assessment – the keystone for the future of toxicology
The basics of toxicology are constantly being reconsidered, and the approach to risk assessment is therefore constantly being put to the test, because, as William Osler is cited in this publication, “Medicine (toxicology) is a science of uncertainty and an art of probability“.
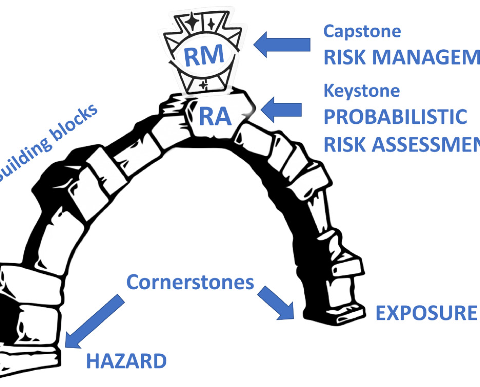
In this recent paper, the team around Thomas Hartung (Johns-Hopkins University/University of Konstanz) has shown that for improved toxicology we should rather work with a “Probabilistic Risk Assessment” approach. This is also or especially important for new materials, because with these there are particularly often gaps in knowledge, uncertainties in risk assessment due to conflicting data and the most diverse hypotheses and strategies of the various stakeholders. In the publication, various models are presented that are applicable for this type of risk assessment and for some of which corresponding software is also available to perform calculations for the respective exposure scenarios. In the examples for this approach, a paper by Jacobs et al. (1) is also cited here, who had applied the case to silica in food. They concluded that after taking all uncertainties into account and using all available data, the margin of safety has not yet been exceeded by far using silica in various food products. In 2017, an international group of experts applied this method to Titanium dioxide in seven different exposure scenarios and concluded no increased risk to humans, as the probability of exceeding the safety limits is vanishingly small (2).
The suggested approach by Johns Hopkins University is thus a good indication to adopt this method in order to be able to make a reasonable risk assessment for new, innovative materials even in the presence of uncertaintie.
Further literature:
- Jacobs, R., van der Voet, H., and Ter Braak, C.J. (2015). Integrated probabilistic risk assessment for nanoparticles: the case of nanosilica in food. J Nanopart Res 17, 251
- Tsang, M.P., Hristozov, D., Zabeo, A., Koivisto, A.J., Jensen, A.C.O., Jensen, K.A., Pang, C., Marcomini, A., and Sonnemann, G. (2017). Probabilistic risk assessment of emerging materials: case study of titanium dioxide nanoparticles. Nanotoxicology 11, 558-568
Original publication:
Maertens, A., Golden, E., Luechtefeld, T.H., Hoffmann, S., Tsaioun, K., and Hartung, T. (2022). Probabilistic risk assessment – the keystone for the future of toxicology. ALTEX 39, 3-29
Weitere Spotlights
Spotlight June 2021: Endotoxin – the reason for false-positive toxicity testing for advanced materials?
Advanced materials, but also nanomaterials are closely examined to determine whether they trigger biological effects that could be harmful to humans and the environment before they are used in products. This also includes such materials as titanium dioxide, which has been used in a wide variety of products for more than 50 years. A particularly […]
Read moreSpotlight April 2022: A new risk assessment of nanomaterials in 3D printing is needed
The use of nanomaterials in 3D printing has great potential. Due to the properties of nanoscale materials, many requirements can be implemented in 3D printing. However, these unique properties based on the size of the particles also lead to the need for new risk assessments. This is because if the nanoparticles are released in the […]
Read moreSpotlight November 2022: Photonics in nature and bioinspired designs
Science has always taken nature as a model and imitated it. If you look at the field of photonics, i.e. the use of optical technologies for information processing, transmission or storage, the colorful examples in the animal and plant world are perfect basic drawers for technical applications. While colors in nature are used either for […]
Read moreSpotlight February 2023: New sustainable and promising method to give cotton textiles an antiviral and antibacterial finish
Textiles have been the subject of research into functionalization for many years, especially also to repel bacteria and viruses. Since the development of nanotechnological processes, there have been many attempts to incorporate UV protection with nano-titanium dioxide, or to provide textiles with anti-bacterial properties with nanosilver (see cross-sectional text “Nanoparticles in Textiles”). But nanosilver has […]
Read more


