Our gastro-intestinal tract is highly specialised for the uptake of different materials (food), their digestion, and the delivery of the nutrients via the blood to the organs. There is also the possibility for nanomaterials to cross the gastro-intestinal tract barrier. However, this only relates to a very small amount of nanomaterials that are considered to be unproblematic.
Food and Gastro-Intestinal Tract
Our body depends on the daily uptake of food and water. In emergencies, we can survive without food for several days to a few weeks but only if sufficient water is imbibed. Without water, our body can live for only a few days. Our solid food components contain a combination of plant and animal products. These consist mainly of carbohydrates, fats, proteins, DNA, as well as minerals and fibres.
The different consecutive parts of our gastro-intestinal tract have the following purposes:
-
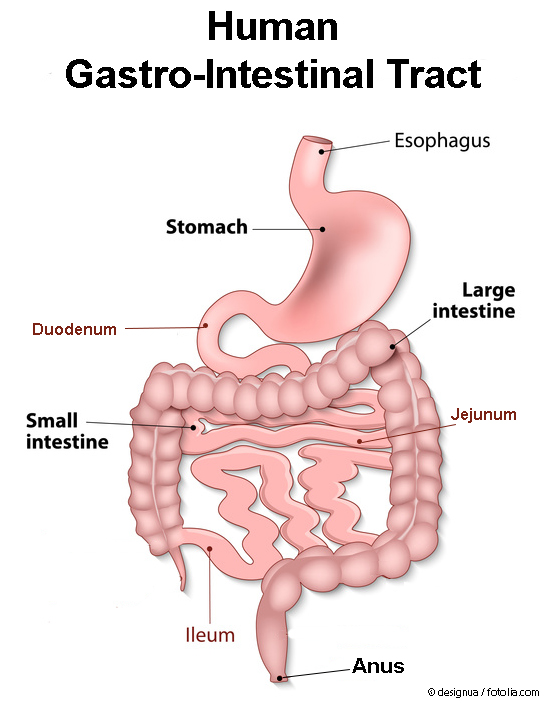
Stomach – The acidic gastric juice and the kneading movements of the stomach musculature enable the production of food chyme. In addition, bacteria and other unwanted biological products are inactivated.
- Small intestine – The main purpose of the small intestine is the processing of the food and the uptake of various nutrients into the body
- Duodenum – Neutralisation of the acidic chyme and addition of bile and digestive enzymes, so that the fatty components are distributed more homogenously and become more water-friendly in order to improve the digestion.
- Jejunum – Separation of the food contents into single components and active uptake of proteins, fats, sugars, and vitamins.
- Ileum – Active uptake of residual nutrients (for example vitamin B12) and processing of the chyme into the large intestine.
- Large intestine – Solidification of the digested food („faeces”) and recuperation of water and essential electrolytes (salts, ions)
- Anus – Control of defecation
Many minerals and dietary fibres – the latter consisting mainly of carbohydrates – which are important for the digestive processes as well as for our body exist in particulate form and also can have sizes in the nanometre range.
In addition, synthetic nanomaterials of the following origins may be present in the gastric contents:
- Food, (drinking) water, processed drinks: Food additives such as silicon dioxide may also contain nano-sized particle fractions
- Medications: additives such as silicon dioxide or titanium dioxide may also contain nano-sized particle fractions
- Medical administration of nanomaterials in form of contrast agents
- Mucus from the lung: as a result of cleaning processes in the lung inhaled nanomaterials may be contained in the lung mucus and be swallowed involuntary (see also “Nanoparticles and the Lung“)
According to present law, the producers are obligated to list all synthetic components in food as well as the general composition of all „processed foods” on the package. Since December 2014, in the EU all food additives in the nanometre size range have to be specifically labelled with the addition of „(nano)” in the list of ingredients (see FAQ „labelling nanomaterials“).
Nanomaterials in the Stomach
The stomach possesses an especially thick mucous membrane which in addition is surrounded by connective and muscular tissues. For this reason, with the exception of very few substances such as alcohol, no materials – including nanomaterials – can cross this barrier. The gastric juice, however, may enable the partial or even total dissolution of certain metals (copper or silver) or some metal oxides (such as copper oxide or zinc oxide) that may be contained in form of nanomaterials. For this reason, simulation experiments with artificial gastric juice are important for these materials.
Nanomaterials in the Intestine
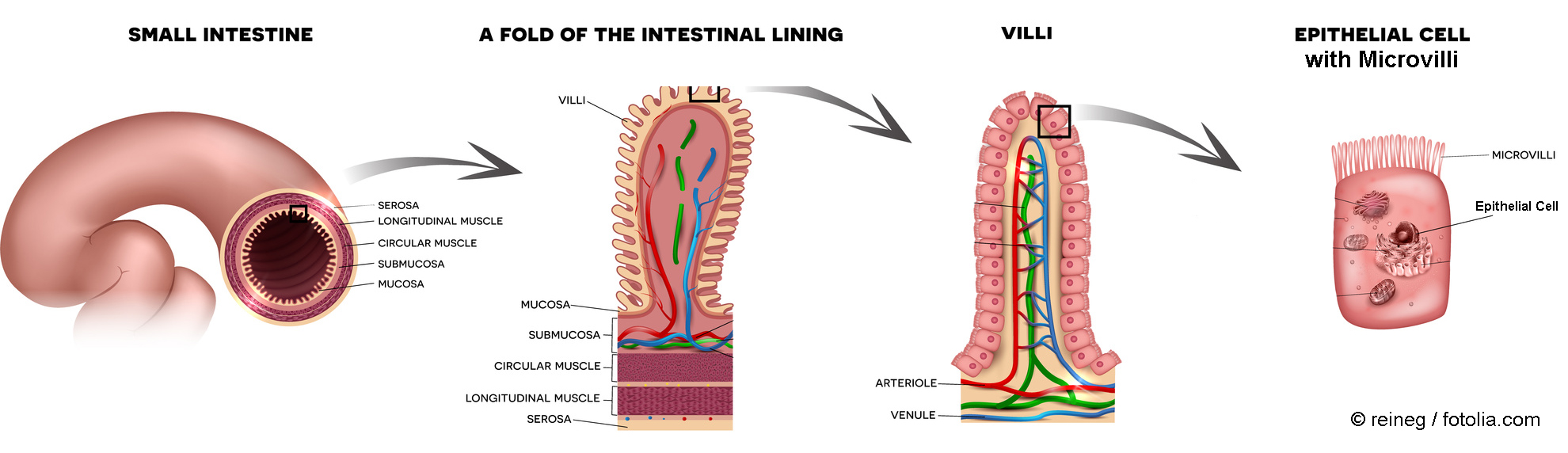
The human intestine has a length of about 6 meters and a very large surface area of about 100-200 m2 including the villi. The villi are equipped with a large number of microvilli, enhancing the surface to several hundred square metres, which is comparable to the area of two tennis fields. This huge area permits the uptake of many low molecular weight substances such as nutrients, vitamins, and certain drugs but also of poisons and other unwanted materials. It is assumed that about 1012 to 1014 nanoparticles per day reach the gastro-intestinal tract via the food [1].
Controlled studies with volunteers demonstrated that micro- und nano-sized titanium dioxide particles are taken up very poorly or even not at all via the gastro-intestinal tract. In the case of silver nanoparticles, only small amounts up to a maximum of 10 % of the previously administered quantity were taken up without exhibiting any physiological effects. Furthermore, nanoscale silicon dioxide particles have been used for more than 50 years as important excipients in tablets as well as thickening agents in different foods. Likewise, nano-sized barium sulphate, which is used in medicine as a contrast agent for X-ray diagnostics, is not taken up by the body and is excreted unchanged [2-5].
Easily soluble nanoparticles do not cause a specific particle-related toxicity, but like any metal salt, the dissolution can result in an enhanced exposure to the respective metal and (at higher dosages) induce the same toxic effects (for instance copper poisoning). Animal models showed that nanoparticles often accumulate in certain areas of the intestine, the so-called Peyer’s Patches. These “islands of the immune system” are present in the entire small intestine and currently being discussed as possible entry points for nanoparticles into the body. However, in all previous studies, the absorbance levels for the nanoparticles were in the very low percent or per mille range [6-11].
Anything that is not needed by the body usually remains in the intestine and is later excreted via the faeces. Therefore, nanoparticles that have been ingested via the food are treated as fibres and excreted at the end.
Damaged Intestinal Barrier
If the intestinal barrier is damaged or functionally impaired for example by inflammatory diseases such as Crohn’s disease, Ulcerative colitis, the uptake rate for nanoparticles might be higher compared to the healthy gastro-intestinal tract. This effect of a higher accumulation of nanoparticles in inflamed intestinal areas on the other hand may possibly be used in medical therapy: Nanoparticles made of poly-(lactic-co-glycolic acid) as well as silicon dioxide therefore are in development as drug carriers for the local therapy of such inflammatory intestinal diseases. [12-15].
The gastro-intestinal tract is used to cope with „foreign materials”. Nevertheless, the possibility exists that nanoparticles contained for instance in food can cross the intestinal wall and enter the body. However, this relates to only very small amount of nanomaterials that are generally regarded as harmless [11,16,17].
- Lomer, MC et al. (2004), Br J Nutr, 92(6): 947-955.
- Kumar, N & Kumbhat, S (2016), Essentials in Nanoscience and Nanotechnology. Wiley & Sons, pp. 441. ISBN:9781119096122
- Jones, K et al. (2015), Toxicol Lett, 233(2): 95-101.
- Munger, MA et al. (2014), Nanomedicine, 10(1): 1-9.
- Scherer, D et al. (1993), J Drug Target, 1(1): 21-27.
- Nefzger, M et al. (1984), J Pharm Sci, 73(9): 1309-1311.
- Kreuter, J et al. (1989), Int J Pharm, 55(1): 39-45.
- Jani, PU et al. (1992), Int J Pharm, 86(2-3): 239-246.
- Hillery, AM et al. (1994), J Drug Target, 2(2): 151-156.
- Araujo, L et al. (1999), Int J Pharm, 176(2): 209-224.
- Yada, RY,et al. (2014), Compr Rev Food Sci Food Saf, 13: 730–744.
- Lamprecht, A et al. (2005), J Pharmacol Exp Ther, 315(1): 196-202.
- Schmidt, C et al. (2013), J Control Release, 165(2): 139-145.
- Moulari, B et al. (2008), Biomaterials, 29(34): 4554-4560.
- Hua, S et al. (2015), Nanomed – Nanotechnol Biol Med, 11(5): 1117-1132.
- Frohlich, E & Roblegg, E (2012), Toxicology, 291(1-3): 10-17.
- Bergin, IL & Witzmann, FA (2013), Int J Biomed Nanosci Nanotechnol, 3(1-2): 163-210.
 >
>