MBBT and TBPT are the abbreviations for substances used in sunscreens to filter out the ultraviolet component of light and thus prevent sunburn. They are examples of so-called organic UV blockers. MBBT and TBPT convert UV light into heat. They do not occur in nature. Instead, they are always produced by the chemical industry.
How can I come into contact with this material?
Sunscreen protects skin from UV radiation @ juefraphoto-stock.adobe.com
If you apply sunscreen to your skin or use cosmetics with UV blockers, they could contain one of the substances MBBT, TBPT, or both. You can check this by looking at the list of ingredients, which is usually printed on the back of the packaging. However, MBBT and TBPT are commercially available under a variety of different (brand) names.
Another possibility of encountering MBBT and TBPT is in bathing areas through washed-off sunscreen in the water. However, since MBBT and TBPT dissolve very poorly in water, they are more likely to remain in the sediment, i.e., the soil of the aquatic environment. MBBT and TBPT have been detected in aquatic sediments and on beaches.
Is there any risk from this material to humans and the environment?
MBBT and TBPT are approved for use in cosmetics by the responsible authorities, both in their bulk form and as nanomaterials. They are used in sun creams and cosmetics with UV protection. In sun creams they are allowed to have a maximum concentration of 10% of the total volume (i.e., 10 ml per 100 ml of cream). MBBT and TBPT are not expected to enter the body during the usual application of sunscreens on the skin, as the skin is a good barrier against these substances. Negative effects of MBBT and TBPT from sunscreens have not been demonstrated to date. On the other hand, MBBT and TBPT protect against skin cancer.
Washed-off MBBTs and TBPTs from sunscreens dissolve only very poorly. Since they are also hardly degraded by natural processes, they remain in the natural environment for a long time. In bathing lakes, organisms living on or in the bottom of the lakeshore could therefore absorb MBBT or TBPT. So far, however, studies have not demonstrated any negative consequences of such uptake.
Conclusion
MBBT and TBPT are so-called organic UV blockers. They convert harmful UV light into harmless heat. So far, there are no studies showing that MBBT or TBPT could be harmful to humans or the environment. However, MBBT and TBPT are poorly biodegradable. Currently, it is still unclear as whether this has consequences for humans and the environment.
By the way
MBBT and TBPT are used as substitutes for the inorganic UV blockers titanium dioxide and zinc oxide. Together with other substances not discussed here, they are responsible for the sun protection factor in sun creams and protect us against skin cancer.
Properties and Applications
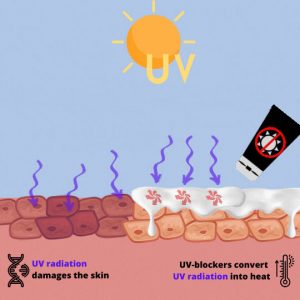
UV-blockers convert UV radiation into heat @ SE
MBBT (Bisoctriazole, belongs to the group of benzotriazoles) and TBPT (Tris-Biphenyl Triazine) are carbon-based compounds used in commercial sunscreen products (cremes, lotions, gels, sprays) and UV blocker containing cosmetics (e.g., in day care creams, make-up, lip balms, hair care products).
The structures of both molecules allow a very efficient absorption of ultraviolet (UV) radiation (both UV-A and UV-B). They convert the UV light into heat without changing their structure or losing effectiveness. Moreover, they are capable of reflecting and scattering UV light. These properties make them very efficient protective agents against UV radiation. The compounds are used as a so-called organic UV-blocking agent in sunscreens to protect human skin from UV radiation-initiated damage. Organic UV blockers are often used instead of inorganic UV-blockers such as nanoscale titanium dioxide (TiO2) .
For application in cosmetic or sunscreen products the UV absorbers need to be suspended in oil or water. Since MBBT and TBPT are almost insoluble, they are used as fine, nanoscale particles since 2009. For this reason, both compounds must be labelled in the product information (ingredient list) as “(nano)” according to the European Cosmetics Legislation. Like small inorganic particles, the UV protection of MBBT as well as TBPT is enhanced by scattering and reflection of the UV light due to their small size .
The discussed organic UV-blockers in the nanomaterial form were approved in Europe for their use as UV blockers in cosmetics and personal care products in 2014 and 2018 respectively, they show the highest efficacy of any commercial product.
Origin and production of UV blockers
TBPT and MBBT do not occur naturally. They are therefore produced via chemical synthesis and in most cases with the aid of a catalyst. TBPT is then usually ground into fine particles in water using dispersants to prevent agglomeration and thickeners to prevent sedimentation. The milled particles can then be easily added to cosmetic products .
Further Information
- European Commission (2021). Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (01/10/2021)
- M. Sohn, S. Krus, M. Schnyder, S. Acker (2021). Performance, Safety and Sustainability – All in Tris-Biphenyl Triazine. OFW Journal 7+8/21, Volume 147, Thannhausen, Germany, August 06, 2021
The two organic UV-blocker MBBT and TBPT are used in the form of micro- and nanoparticles in sunscreens (creams, lotions, gels, pump sprays) and cosmetics with UV-Blockers (e.g. in day care creams, make up, lip care sticks, hair care products). Therefore, an application-related exposure of humans is only given via the skin.
Everyday Contact

Woman with product in a drugstore ©Robert-Kneschke-stock.adobe.com
The use of sunscreens against UV radiation exposes the human skin to various substances. A typical mixture of a sunscreen cream contains the actual active ingredients, in this case MBBT and TBPT, which reflect or absorb the UV radiation and convert it into heat, plus a number of excipients, skin care agents and fillers. After the two organic UV-blockers MBBT and TBPT had already been used as larger particles for many years, they have also been used in the nano size since 2009. This also required a new approval, which was granted in 2014/2018 for the various compounds. In contrast to the inorganic UV-blockers titanium dioxide and zinc oxide, the particle size has little to do with the effect, because the absorption of UV light is an effect of the organic compound. However, nanometer-sized particles in the cream can be distributed much better and more evenly on the skin than larger particles and thus have an improved effect. Therefore, increased attention must be paid to a possible absorption into the body and an effect in the body.
Consumer Situation
When using sunscreens and other personal care products, which may contain up to 10% by volume of the two substances, the organic UV-blockers MBBT and TBPT are distributed over a large area on the skin, therefore the skin is the exposed organ.
Situation at the Workplace
For the two organic UV-blockers MBBT and TBPT there is no specific size-dependent assessment for the workplace in the production and further processing of both substances. As dry particles that are almost insoluble in water, they fall under the general dust limit value (see ECETOC Report for occupational exposure limits).
Human exposure occurs mainly during application to the skin. Here, the two organic UV-blockers MBBT and TBPT are intended to protect the skin from harmful UV radiation and thus prevent DNA damage and possible skin cancer.
Further Information
Organic UV-Blocker such as MBBT or TBPT are used in micro and nano size in sunscreens or cosmetic products with UV protection and can be detected in water. This concerns lakes, rivers and seas.
Release of organic UV-Blocker
Organic UV-Blocker (MBBT or TBPT) like inorganic UV-Blocker (e.g. titanium dioxide or zinc oxide) can enter bathing waters directly by washing sunscreen off the skin during bathing or can enter wastewater during showering and subsequently enter rivers or water bodies through the discharge of treated water .

Arm is covered with sunscreen ©lesterman -stock.adobe.com
According to EU regulation, since 2014 the organic UV-Blocker MBBT and TBPT may also be used in sun creams in nanoform in concentrations of up to 10% of the volume (e.g. 10 ml per 100 ml sun lotion) . Due to the large manufacturing volumes and the persistence of particulate organic UV-Blocker, MBBT has been detected in rivers and seas, e.g., in Germany and India .
Studies have detected MBBT in the North Sea, the Baltic Sea and in rivers flowing into both seas .
MBBT is the most frequently detected organic UV-Blocker in the Baltic Sea (approx. 40% of the total amount of the 22 different organic UV-Blocker detected). In the North Sea and the tributaries of the Elbe and Rhine rivers, the amount of MBBT varied between 5 and 20% among several organic UV-Blocker studied .
In further studies on beaches of Gran Canaria, MBBT was also found. Because of its persistence, organic UV-Blocker would probably be found in other locations as well, but there are provable studies only for the locations listed here (Baltic Sea and Gran Canaria) .
Regarding nanoscale TBPT, there is currently no information on its occurrence in the environment. However, numerous other non-nanoscale organic UV-Blocker have been detected in the waters studied .
Released amount of organic UV-Blocker
Studies using sediment samples have detected MBBT in the nanogram range in the Rhine, the Rhine-Meuse Delta, and the Baltic Sea .
Over time from 2005-2013, neither an increase nor a decrease in MBBT concentrations in water could be detected in two German rivers .
This could be due to several reasons. A constant concentration either suggests that a constant amount of MBBT is being discharged into and out of rivers, or that less is being discharged but MBBT is very persistent and therefore can be detected over a longer period of time.
Currently, very few studies exist on the distribution of MBBT or TBPT. However, there are other studies on the occurrence in the environment of structurally similar organic UV-Blocker, so that these can serve as a comparison. For example, the product UV 329, which like MBBT belongs to the substance class of benzotriazoles, could be detected in Indian rivers in the nanogram range per liter . Even though UV 329 is not a particulate UV-Blocker in nanoform, the concentration detected in rivers in India is of the same order of magnitude as MBBT in German rivers. This demonstrates the worldwide use of various organic UV-Blocker.
Due to their application as sunscreens, organic UV-Blocker such as MBBT or TBPT are found in many waters. They accumulate in the sediment. The amounts detected at the present time are not hazardous to environmental organisms according to current knowledge, but may accumulate in marine organisms due to their persistence.
Basically, two exposure scenarios with the organic UV-Blockers MBBT or TBPT in micro- or nanoform are conceivable for humans: firstly, at the workplace through inhalation of dust particles during processing or, secondly, during application as a cosmetic care product on the skin. So far, there is no evidence that the organic UV-blockers enter the human body in significant quantities.
Uptake via the lung
The two substances MBBT and TBPT are practically insoluble in aqueous environments and are classified as inert, i.e. their interaction with the environment is negligible. Therefore, no studies are available on specific exposure at the workplace, apart from dust regulation, which must be always observed in the workplace. Thus, the two particulate substances at the workplace fall under the legal regulation of GBP (see cross-cutting article - granular biopersistent dust particles).
Uptake via the skin
The skin is the exposed organ, as these two organic UV-blockers are used in skin creams. Even when the micrometer-scaled MBBT particles were approved, studies were conducted to analyze penetration of the particles through the skin. In one study, approximately 200 nm organic UV-blockers were compared with 60 nm titanium dioxide particles. For both materials, the organic as well as the inorganic UV-blockers, no penetration through the skin was detected . Two years later, another study confirmed this result for both the organic and inorganic UV-blockers. Several organic compounds were used, including the MBBT, and both inorganic nanoparticulate materials, titanium dioxide and zinc oxide. No skin penetration was observed for the two inorganic nanoparticles and MBBT . Again, two years later, the result of the first studies was confirmed with an improved detection method . Additionally, after application of the organic UV blocker MBBT in nanometer size, no particles could be found in deeper layers of the epidermis, so that no uptake via the skin could be detected .
Uptake via the Gastro-Intestinal tract
Since there are no applications that would suggest absorption via the mucous membranes of the gastrointestinal tract, this route of absorption has not been investigated.
The two organic UV-blockers MBBT and TBPT are considered like normal dust in the workplace and fall under the legal regulation of GBP dust. Due to the direct application on the skin, an uptake there would be most likely, but it could be shown in several studies that no uptake of both the organic and the inorganic UV-blockers independent of size via the skin is detectable.
There is little detailed knowledge on the uptake and mechanisms of action of organic UV-Blocker such as MBBT or TBPT on environmental organisms. Most findings are available on MBBT. However, toxic effects have been observed in aquatic organisms for structurally similar organic UV-Blocker.
Release of organic UV-Blocker in environmental organisms
Organic UV-Blocker such as MBBT, which are present in the final product as particles, are taken up by freshwater and saltwater organisms via water or sediment. Transfer through the food chain from edible fish to humans has not yet been documented for MBBT or TBPT, but seems likely due to the persistence of organic UV-Blocker. There is currently no information on potential concentrations and effects .
Toxicity of organic UV-Blocker in environmental organisms
The amounts of MBBT detected in the Baltic and the North Sea are not hazardous to marine saltwater organisms (e.g., bream). However, long-term accumulation in these organisms cannot be excluded .
Sediment and fish (bream) samples were collected at eleven different locations for five German rivers (e.g., Saar and Rhine). Among the substances analyzed, MBBT was detected most frequently in sediment. In fish, the uptake was so low that MBBT could not be detected even in the liver of the fish .
Assessment of the effects of organic UV-Blocker in environmental organisms
Due to the input of organic UV-Blocker into rivers and seas, sediment-dwelling, aquatic freshwater and saltwater organisms are particularly exposed, for example, at so-called hot spots (such as beaches), where high concentrations of these substances are to be expected . Since there are currently no publicly available laboratory studies on concentration-response relationships of MBBT, no conclusions can be drawn about the effects of MBBT amounts in the environment. However, it is known that UV-Blocker are difficult to degrade and can therefore accumulate in the environment.
Organic UV-Blocker such as MBBT enter water bodies due to their use as sunscreens and other cosmetic products and can thus also be taken up by environmental organisms. Further findings on possible toxic effects and mechanisms of action for environmental organisms are not yet available for either MBBT or TBPT.
Although a few studies have found an inflammatory effect in the lungs, the organic UV-blockers MBBT and TBPT in any particle size are considered safe because absorption into and through the lungs is unlikely. None of the substances is absorbed through the skin, so no effect can be caused there either.
Distribution and effects in the body
As mentioned above, the substances MBBT and TBPT are not absorbed through the skin and exposure via the lungs or gastrointestinal tract is considered unlikely. Therefore, no adverse effects are expected for humans under normal conditions . An early study had already investigated whether MBBT could possibly have a hormonal effect and would thus be counted among the „endocrine-disrupting substances“. However, a hormonal effect could definitely be excluded in this study .
Nevertheless, various studies have to be carried out on a legal basis for the authorization of the substances. All the following descriptions are taken from the dossier of the Scientific Committee on Consumer Safety (SCCS) of the European Commission . In laboratory tests described there, an acute inflammatory reaction was observed after inhalation of a high concentration of the substance MBBT (high exposure concentration for 4 hours), without subsequent toxicological effects. For the nanoform, this dossier also attests no absorption via the skin. This applies to healthy but also to pre-damaged skin. Furthermore, no mutagenic or carcinogenic effects were detected in the cell culture and animal tests. Although a severe inflammatory reaction in the lungs occurred only under the exposure conditions in the laboratory test, conditions that do not occur in the actual use of the substances in the sunscreens, the Scientific Committee on Consumer Safety has advised against using these two substances in applications that could cause exposure of the lungs.
With regard to sunscreens, there are repeated indications that they can trigger allergic reactions in sensitive individuals and thus the so-called "Acne aestivalis or Mallorca acne" would also be such an effect of the UV-active substances. However, two factors play an important role: first, it is the genetic predisposition that makes certain people more sensitive to sunlight. Then, in addition, there are the ingredients of sunscreens, which can intensify such an effect of the solar radiation. However, this explicitly does not include the organic or the mineral UV blockers. It has been shown that MBBT, for example, does not induce such an effect, but the glucosides used as surfactants in the creams, which may be present as accompanying substances, do .
Based on the above and other studies, the two substances MBBT and TBPT were considered safe for use as UV-blockers in any particle size in cosmetics and skin care products. An application that would lead to lung exposure has been explicitly excluded by the SCCS committee.
Further Information
- European Commission: Environment - Endocrine Disruptors (last accessed 11/2021)
Due to the small number of studies on MBBT and TBPT, it is currently not possible to make any sound statements on the distribution of these organic UV-Blocker in the environment. However, the materials are difficult to degrade in the environment, they remain almost unchanged and are distributed in aquatic and marine ecosystems and in the corresponding sediments.
Transport

Sandy beach ©dimakp-stock.adobe.com
MBBT has been shown to accumulate in sediment at the bottom of water bodies .
Transformation
Various organic UV-Blocker, including MBBT, accumulate in the environment. Thus, no degradation could be detected in studies over a period of 100 days .
Organic UV-Blocker are difficult to degrade, they accumulate mainly in sediments.
 >
>