FAQs
Definitions
No, they are not. The size of a material alone does not determine its toxicity. Most substances exerting hazardous effects in the nano-size show the same toxic effects for larger sizes / bulk material. However, the exposure and distribution within organisms may differ, e.g. nanomaterials can reach deeper regions of the lung upon inhalation whereas this is not possible for larger particles.
Nanomaterials have unique properties, which make them so interesting for many novel applications. At the same time, concerns arose, whether these new properties lead to unexpected hazard towards humans and the environment. Many research activities were performed to tackle this question. So far, no unique mechanisms of hazard exclusively occurring for nanomaterials were discovered. The toxicity of a substance is rather determined by the type of material than by its size.
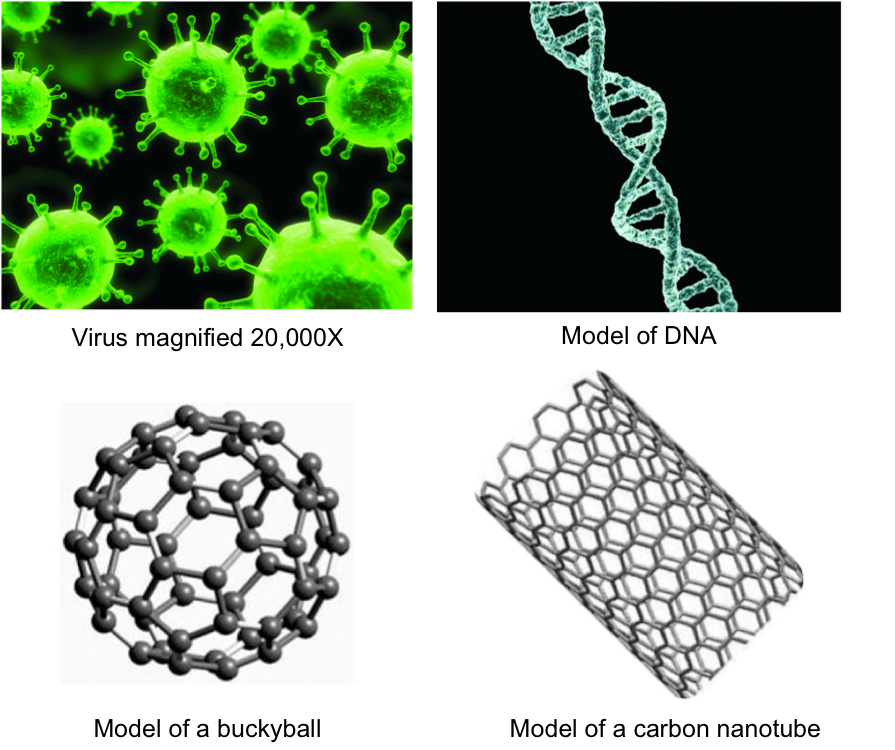
Size comparison Virus DNA Fullerene CNT
The term „nano“ derives from the greek word nanos, dwarf. A nanometer is one millionth of a milimeter. It is equal to 1/1,000,000,000th or one-billionth of a meter. When things are this small, you can’t see them with your eyes, or a light microscope. Objects this small require a special tool called electron microscope (EM) or scanning probe microscope (SEM).
Nanoparticles range in size from 1 nm to 100 nm.
All these naturally and synthetic things are on the nanometer scale: Virus (30-50 nm), DNA (2.5 nm), buckyballs (~1 nm in diameter), CNT (~1 nm in diameter).
UPDATE!
In 2011, the European Commission published a first common definition of the term “nanomaterial” (2011/696/EU). This was updated in 2022:
‘Nanomaterial’ means a natural, incidental or manufactured material consisting of solid particles that are present, either on their own or as identifiable constituent particles in aggregates or agglomerates, and where 50% or more of these particles in the number-based size distribution fulfil at least one of the following conditions:
- one or more external dimensions of the particle are in the size range 1 nm to 100 nm;
- the particle has an elongated shape, such as a rod, fibre or tube, where two external dimensions are smaller than 1 nm and the other dimension is larger than 100 nm;
- the particle has a plate-like shape, where one external dimension is smaller than 1 nm and the other dimensions are larger than 100 nm.
- In the determination of the particle number-based size distribution, particles with at least two orthogonal external dimensions larger than 100 μm need not be considered.
However, a material with a specific surface area by volume of < 6 m2/cm3 shall not be considered a nanomaterial.
https://ec.europa.eu/environment/chemicals/nanotech/pdf/C_2022_3689_1_EN_ACT_part1_v6.pdf
A material is called a nano-object when one, two or three external dimensions of it are present in nanoscale. This includes nanoparticles, i.e. nano-objects with all three external dimensions on the nanoscale. Nano-platelets are nano-objects with one external dimension on the nanoscale, and two much larger external dimensions. Nanofibers have two similar external dimensions on the nanoscale, and a third external dimension that is much larger than the other two dimensions.
These definitions were developed in 2008 by the Technical Committee ISO/TC 229 “Nanotechnologies” in collaboration with the Technical Committee CEN/TC 352 “Nanotechnologies”.
Nanoparticles can be of different chemical nature. Both inorganic and organic nanoparticles are known. They can consist of only one element, i.e. metal or carbon or of compounds like oxides, nitrides, etc. Nanocomposites are understood to be composite materials that have at least one component in the form of a nano-object. Nanoparticles often build clusters of aggregates or agglomerates. By contrast to aggregates, agglomerates can be ground into the primary grains through optimal mixing. Therefore, their shape can be very inconsistent and they may take a wide variety of forms which has considerable influence on their properties. In principle, because of their enormous surface-to-mass ratio nanoparticles behave completely different than larger composites.
Workplace
If protective technical measures against unintentional release of nanomaterials at the workplace are NOT sufficient, the German Social Accident Insurance (DGUV) recommends the use of personal respiratory protection (filter class P3 or P2). When selecting the personal respiratory protection device, the previous risk assessment for the work place has to play a major role. If the wearing of a breathing mask is required, it has to be fitted tightly to the face or head. Likewise, current time limits and regulations for the wearing of breathing masks have to be respected. Besides personal protection, teaching and training of employees on the correct use is important too.
- Further information on this topics are provided on the DGUV website or in our FAQ on “Who is responsible for occupational safety in the handling of nanomaterials, and where can I find relevant information?“
Safety at the workplace is the responsibility of the employer. He is responsible for all basic trainings, assessment & management of risks and potential hazards at the workplace as well as the implementation of the associated protective safety measures. The employee has to act safely in accordance with the training and has to inform the employer about any possible further risks. The European legislation on worker protection also applies to nanomaterials. Similarly to the handling of other hazardous substances, the same prevention measures (“STOP”) following the hierarchy of control are relevant:
- Substitution
- Technical control measures at the source
- Organisational measures
- Personal protection equipment
Further information on this topic of nanomaterials and occupational health & safety can be found on the websites of the appropriate authorities, insurers or the European Agency for safety and health at work (EU-OSHA).
Germany
- Federal Institute for Occupational Safety and Health (BAuA) – Nanotechnology
- Nanoportal of the German Social Accident Insurance (DGUV) (in GERMAN)
- German Social Accident Insurance (DGUV) – Ultrafine aerosols and nanoparticles at the workplace
Switzerland
- Swiss Federal Office of Public Health (FOPH) – Occupational health protection
- Federal Coordination Commission for Occupational Safety (FCOS)
- Swiss Social Accident insurance (SUVA) (in GERMAN) – Nanopartikel an Arbeitsplätzen
Austria
- Arbeitsinspektion (Bundesministeriums für Arbeit, Soziales und Konsumentenschutz) – Nanomaterialien (in GERMAN)
- Nanoinformation-Portal (in GERMAN)
European Union
- European Agency for safety and health at work (EU-OSHA) – Managing nanomaterials in the workplace
Up-to-now, there are no European occupational exposure limits in place. However, the establishment of such values is an ongoing process. So far, health and safety measures for the handling of nanomaterials are based on the precautionary principle of existing knowledge derived from safety measures for handling chemicals. This includes in particular the avoidance of contact with the particles (exposure) and the use of personal protective equipment (for example, respiratory protection, protective gloves).
In Germany, the Federal Institute for Occupational Safety and Health (BAuA) is dealing with the topic of nanosafety at the workplace; in Switzerland, the Federal Office of Public Health (FOPH) has created a precautionary matrix for synthetic nanomaterials.
Links:
- German Federal Institute for Occupational Safety and Health (BAuA) – Nanotechnology
- Swiss Federal Office of Public Health (FOPH) – Precautionary Matrix for Synthetic Nanomaterials
- Brochure “Safe handling of nano materials and other advanced materials at workplaces” (2015)
- European Precautionary Principle (last update 21.09.2015)
Application/Products
In solid-state batteries, both electrodes and the electrolyte consist of solid material. Although some solid-state batteries are already in use in electric cars or trucks (e.g. in the “Bollore Blue Car”), most are currently still under development.
The advantages of all-solid-state batteries (ASSB) over the common liquid electrolyte batteries are the replacement of the (usually highly flammable) liquid electrolyte by a solid electrolyte. Furthermore, solid-state batteries have a higher energy density and a faster charging capacity, which is an important property for the rise in electromobility.
Different materials such as polymers, metals and ceramics are used in various combinations for solid-state batteries.
Liquid electrolyte batteries have been used for many years and are currently the most commonly used batteries, e.g. lithium ion batteries (LIB) in mobile phones. In liquid batteries, the electrolytes are present in dissolved form, but the electrodes are made of a solid material. The liquid electrolytes are usually highly flammable, which is often seen as a disadvantage. Therefore, solid-state batteries are currently being developed in which the electrolytes are in solid form.
Liquid electrolytes are mostly water-based salt solutions consisting of organic and inorganic acids. However, simple saline solutions can also be used.
There are a variety of rechargeable batteries or secondary batteries, which can be roughly divided into liquid electrolyte batteries (also called wet cell) and solid-state batteries. Generally, they consist of 2 electrodes and an electrolyte, each of which can be made of different materials.
The majority of batteries in use today and in the near future are liquid electrolyte batteries, mainly lithium ion batteries.
Some solid-state batteries are already in use, most others are currently in basic research. Materials used are, for example, sodium, magnesium or aluminium.
Conventional solar cells are often made of silicon dioxide, which is used in amorphous or crystalline form. It takes a lot of know-how and money to produce it, and the yield of light energy conversion in commercial products is less than 25% [1]. Therefore, solar cells are also made from other elements. Among the most prominent representatives here are GaAs (gallium arsenide), CdTe (cadmium tellurium) and CIGS (copper indium gallium selenium) solar cells. These elements are controversial, as some are e.g. toxic, only difficult to obtain from socially / ethically acceptable sources and usually very expensive.
Therefore, sustainable alternatives are being sought: perovskite solar cells could be cheaper to produce and the starting materials easier to obtain. However, they also contain toxic elements (status 2022). Intensive research is therefore being conducted into less toxic perovskite solar cells. Good luminous efficiencies have already been achieved in the laboratory, and in particular the possibility of building semi-transparent cells could lead to very high yields in light energy conversion if perovskite and conventional solar cells are arranged one above the other (tandem solar cell or multi-junction solar cell).
Further infomation
- US National Renewable Energy Laboratory (NREL) – Best Research-Cell Efficiency Chart
Silver nanoparticles impart antimicrobial properties to plastic packaging materials and can prevent the growth of food-damaging microorganisms via the targeted release of silver ions. There is a possibility that, in addition to silver ions, silver nanoparticles are being released from the packaging and migrate into the food. From a research perspective, this issue has not yet been conclusively resolved and is being further investigated. Nanosilver, one of the most prominent examples, which is commonly used in the USA, Asia or Australia, is currently not allowed to be used in plastic food packaging in the EU.
- For more information on this topic, please refer to the article in our cross cutting section “Nanomaterials in food packaging”
Currently no intentionally produced nanomaterials are used in food & feed applications in the EU. The occurring nanomaterials are byproducts of the production process of approved food additives such as silicon dioxide. In Asia and North America however, nanomaterials are used specifically in food products, e.g. for the encapsulation of vitamins. Based on the majority of existing studies, current approved food additives pose no health risk for humans. Since EU law allows only harmless (safe) additives to be used in food, every food additive has to be tested and legally approved prior to any products’ application. This decision is based on latest developments in science and technology and is therefore constantly under revision.
- For more information on this topic, please refer to the article in our cross cutting section “Nanomaterials in Food”
Acrylic resins are particularly durable synthetic resins and are used in various adhesives, paints and coatings. Before curing, they are present as monomers in liquid form and therefore not nanoparticles. Due to the wide range of possible applications, they may contain various additives e.g. consisting of nanoparticles. However, there is no health risk associated to nanoparticles containing acrylic resins as the nanoparticles are firmly bound in both the liquid and cured form and cannot be released into the environment.
In many cases, silver threads are woven into the mattress’ cover and the fabric can be additionally impregnated with a silver solution.
The aim of these enhancement methods for textiles is an antimicrobial effect: Under moist or humid conditions, positively charged silver ions are being released by the metallic silver surface exerting their biocidal effects against bacteria, yeast or fungi. This behaviour is independent of size and origin of the silver ions, stemming either from silver salts or from metallic silver particles. However, due to higher specific surface area, silver nanoparticles are able to release much more silver ions, which in turn cause a stronger antimicrobial effect compared to larger particles. At the present state, nanosilver-equipped textiles like those used in mattresses, present no risk or danger to human health.
- Further information on this topic “nano in textiles” can be found in our cross-cutting section!!!
Various studies have shown that nano-enhanced paints are per se not more harmful to the human health than conventional paints. The same preventive precautions apply for these paints during use, processing and the subsequent disposal of the products. Especially in case of handling powdery substances, suitable protective clothing or respirators should be used.
- For further information on this topic “nano in paints“, please refer to our cross-cutting section!!!
In general, the nanosilver particles are firmly embedded in the paint. Pristine nanoparticles are rarely released. Sandblasting experiments with nano-enhanced paints demonstrated that it is possible to remove some nanomaterials from the applied paint albeit only to a very small extent. Once particles are being released, they immediately get in contact with other particles or form agglomerates. The conclusion of no aerosolised nanoparticles being present has been confirmed by other studies as well. Small amounts of silver nanoparticles are non-hazardous for humans. Some environmental organisms like fish are more sensitive to silver. Since current studies showed that the estimated amounts of released nanosilver were below detection limit, it can be assumed that these nanoparticles present no risk for the environment.
- For further information on this topic “nano in paints“, please refer to our cross-cutting section!!!
The Swiss project NanoSafe Textiles has carefully assessed current and future applications of synthetic nanoparticles in textiles and evaluated their potential risks for the environment and humans throughout the complete lifecycle. Based on these results the project team compiled a guideline for the textile industry on how to produce safe, sustainable and economically attractive nano-enhanced textiles.
- The guideline together with further information on this topic “nano in textiles” can be found in our cross-cutting section!!!
Special coatings are used to generate self-cleaning surfaces or anti-graffiti-protection. The coatings are virtually invisible and protect the base material without changing its appearance. Adherence of dirt particles on such smooth, non-sticky and water- repellent surfaces (on facades, windows, walls, vehicles) is very poor which is why they can easily be rinsed off and removed. A universal protection coating does not exist due to the large variety of base materials but the coating is individually adapted to the respective system.
When processing and applying the various coatings, the same protective safety measures as for other paints or varnishes apply like wearing of adequate protective clothing or breathing masks.
After curing / drying the nanoparticles are firmly embedded in the coating. As with nano-enhanced paints, pristine nanoparticles are rarely released under environmental conditions (wind, rain). If some particles are being release, they immediately get in contact with other particles or form agglomerates and no individual nanoparticles occur in the air or in water.
- Further information are provided in our cross-cutting article on “Nanoparticles in paints“!!!
Cleaning polluted water or soil from hazardous substances is time- and cost-intensive. Up-to-now the contaminated medium (water, soil) needs to be removed from the environment, cleaned, and then brought back into the original system. If a clean-up is not possible, the water or soil needs to be treated as waste, and is either burned or dumped in landfills. Here nanomaterials offer two advantages: (1) They can be directly introduced into the contaminated environment and (2) the removal of contaminants is more effective due to the higher reactivity of the nanomaterials.
Examples for these kind of applications have been intensively studied in the BMBF-funded projects Fe-NANOSIT, NanoSan and NAPASAN.
No, to effectively act as inorganic UV-blocker (also called mineral UV-blocker) in sunscreen, the materials either in nanoform or lager just need to form a thin layer on top of the skin. If correctly applied (not on mucous membranes or injured skin) the nanomaterials cannot enter the human body and are washed off the skin surface by sweat or water.
- For further information on this topic “Nanoparticles and the skin“, please refer to our cross-cutting section!!!
Typically nanomaterials used in sunscreens like titanium dioxide or zinc oxide (inorganic uv-blockers) as well as the organic nanoscale UV-blockers MBBT and TBPT are not able to cross a healthy skin barrier and enter the human body if correctly applied (not on mucous membranes or wounds). Even in case of sunburnt skin, the nanomaterials stay in the upper layers (epidermis) of the skin. Therefore, in both cases no internal distribution of nanomaterials via the blood stream is to be expected.
- For further information on this topic “Nanoparticles and the skin“, please refer to our cross-cutting section!!!
Two different groups of UV-blockers are used in sunscreen to protect against UV radiation: inorganic (also called mineral) and organic UV-blocker. Both types often absorb the radiation and release it with less energy (e.g. as heat); however, some of the radiation can also be reflected. Both organic and inorganic UV-blocker can be contained in nanoform. From the first group, there are MBBT and TBPT as organic compounds but as insoluble nanoparticles that absorb UV light. Titanium dioxide or zinc oxide belong to the group of inorganic UV-Blockers. The nanoforms do not leave a white film on the skin, which many users prefer for aesthetic reasons.
- For further information on this topic “Nanoparticles and the skin“, please refer to our cross-cutting section!!!
The main motivation to use nanomaterials in pesticide formulations is to increase the solubility of the active ingredient. This has some advantages over conventional pesticides with regard to controlled and targeted release, protection against the degradation of the active ingredient, and hence overall an increased efficiency for pest control. Collectively, this enables reducing the dose of the active ingredient while achieving comparable or even better performance of the product.
- Read more about this topic in our cross cutting article – “Nanomaterials in plant protection products”!
No, a recovery of nanomaterials from a product is not feasible, because the nanomaterials are often tightly embedded in a matrix and the separation of nanomaterials is technically difficult. However, the majority of nanomaterial-containing products is recycled (e.g. plastics, electronic goods).
- Read more about this topic in our cross cutting article – “Nanomaterials in waste”!
Nanoplastics are particles that comprise various polymers (e.g. PET – Polyethylene terephthalate, PS – Polystyrene). Depending on the definition used they are less than 1 μm or less than 100 nm in size, respectively. Thus, the term nanoplastics does not describe a uniform material. It is important to distinguish between primary and secondary nanoplastics.
Primary nanoplastic particles are intentionally produced and used in various products, such as cosmetics, washing powders, as well as in research and diagnostics. They are often of a defined size or size distribution and usually consist of only one polymer type for a given application.
Secondary nanoplastics are formed in the environment, especially in rivers and oceans, by fragmenting larger pieces of plastic. These are released through the improper disposal of waste into the environment where they are decomposed by the influence of sun, wind and waves into even smaller pieces. After so-called meso- and microplastics emerged from the large pieces, these particles disintegrate into even smaller nanoplastics. Depending on the composition of the waste, nanoplastics consist of a mixture of different polymers.
The main difference between nano- and microplastics is the respective size range. Microplastics are exclusively composed of polymers such as PET (polyethylene terephthalate), PP (polypropylene), PE (polyethylene) and comprise a group of larger particles up to 5 µm in size. An agreed definition of microplastics does not exist yet. Primary microplastics describe all industrially manufactured products e.g. for cosmetics, detergents or drug carriers in medicine. However, the majority is represented by secondary microplastics, which is created in the environment by the fragmentation of plastic waste. By further fragmentation eventually also nanoplastic is formed.
Microplastics is currently being discussed as having potential harmful effects on the environment. Various bodies at national and European level are intensively studying this issue. Studies have confirmed the formation of a nanoscale plastic fraction under environmental conditions but there are currently no studies on their possible negative effects on fauna and flora.
For nanomaterials on the other hand, a definition already exists. Their compositions are not restricted to plastic polymers, but also include metals or carbon-based materials. Thank to extensive research during the last year, knowledge on the possible effects of nanomaterials on humans and the environment has been generated.
Research Activities on the topic microplastics:
Silver has been used since thousands of years to keep water sterile. Nowadays nanosilver is more and more used for this purpose, but it is not directly visible if a refrigerator is coated with nanosilver. Nevertheless, the exposure in this case via the food is very small and has no relevance for our health. By using silver cutlery we take up much higher amounts, but this is still far below the exposure limits for toxicological effects.
For more information about silver, please click here www.nanopartikel.info/en/knowledge/materials/silver/
In food packaging many different materials are used and for nanomaterials many application possibilities exist, thus, there is no general answer. There are surely products on the market, which contain nanomaterials or nanosized coatings. Within the EU materials intended to come into contact with food must be sufficiently inert to preclude any adverse effect for human health (Regulation (EC) No 1935/2004) and this is totally independent of the particle size or the type of material.
See also: http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32004R1935&from=DE
Nanomaterials in textiles can either improve existing properties or introduce completely new functionalities. Nano-enhanced textiles can be dirt- and water-repellent, breathable, conductive and antistatic. They offer protection against UV light, have an increased wear and wrinkle resistance or resistance to stains and can reduce infestation of bacteria or fungi.
Besides these personal advantages, replacing traditional materials with nanomaterials for textile applications can help to reduce their environmental or health-related impact as well as reduce the environmental burden. Less material is needed to achieve the same results and a decreased washing frequency helps saving energy and water.
- Further information on this topic “nano in textiles” can be found in our cross-cutting section!!!
In paints, titanium dioxide (chem. TiO2) is mainly used as a white pigment. Only micrometre sized titanium dioxide causes such white colouring. Nanoscale titanium dioxide functions as UV filter protecting the paints’ binder material. Equally, it degrades organic materials via generation of radicals (photocatalytic actovoty ), which is e.g. used for self-cleaning surfaces.
- For further information on this topic “Nanoparticles in paints“, please refer to our cross-cutting section !!!
In the EU and Switzerland, E-numbers are solely used for labelling purposes on the list of ingredients and are not an indication of any potential hazard. Since EU law allows only harmless (safe) additives to be used in food, every food additive has to be tested and legally approved prior to any products’ application. The decision is based on latest developments in science and technology and therefore constantly under revision. Both, silicon dioxide (SiO2, silica, E551) and iron oxides (E172), are approved for specific applications and are “partly” present as nanoparticles. However, this fraction occurs due to production reasons and is not important for the use as food additive.
Further information :
- European food safety authority (EFSA) : https://www.efsa.europa.eu/en/topics/topic/additives
- EU-Portal Food Additives: https://ec.europa.eu/food/food-feed-portal/screen/food-additives/search
Yes, nanomaterials are being generated during the printing process and released into the air in the form of fine dust. As these respirable dust particles are capable of reaching the deeper regions of the lung, printer and copy machines are nowadays located outside the offices in separate rooms.
- Further information on this topic is provided in our cross-cutting article on “Toner“!!!
Production figures are defined as the mass of an industrially manufactured material based on a certain region and time frame. Unfortunately, general production figures for nanomaterials are not publicly available, but can be obtained in parts in various publications from academia and industry. An overview of the production figures of 10 different nanoobjects can be found in the publication from Picchinno et al. 2012. In general, the industrial production figures range from about 60.000 tons per year for “traditional” nanoobjects down to a few kilograms for special nanomaterials.
- Piccinno, F., Gottschalk, F., Seeger, S. et al. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J Nanopart Res 14, 1109 (2012). https://doi.org/10.1007/s11051-012-1109-9
At the moment, only conditionally: on the one hand, products with the attribute “nano” are advertised in which there is no “nano” at all. On the other hand, there are only a few product groups in which nanomaterials have to be labelled:
According to EU Regulation No. 1223/2009, the following applies to cosmetics: “All components in the form of nanomaterials must be clearly stated in the list of components. The names of these ingredients must be followed by the word “nano” in brackets”. In the list of ingredients, which shows the exact composition on each cosmetic product, the inorganic UV-blocker (also called mineral UV-Filter) titanium dioxide, for example, can be found as “TITANIUM DIOXIDE (nano)”.
Nanomaterials should also be highlighted accordingly in the list of food ingredients. From EU Regulation No. 1169/2011: “All ingredients that are present in the form of technically produced nanomaterials must be clearly listed in the list of ingredients. The designation of such ingredients must be followed by the word “nano” in brackets.” However, as neither additives nor other ingredients in nanoform have been approved and used to date, there are no lists of ingredients in which this information can be found.
The situation is similar with biocides, i.e. products that are used against small and large pests. The EU Regulation No. 528/2012 (Biocides Regulation) applies here. Their packaging would also have to be labelled if active substances were used in nanoform.
Most of such nano-finished sanitary ware is surface-structured and exhibits the dirt-repellent lotus effect that is typical of leaves. This effect helps keep surfaces clean or serves to make them easily cleanable. However, this is not achieved by using nanoparticles or by applying them to the respective surfaces but by treating the latter with chemicals.
Occasionally, some manufacturers may aftertreat the surfaces with nanoparticles. Details should be requested from them directly or from the selling shop. The probability of getting exposed to nanoparticles released from surface-structured products is very low.
Question: “Are silver particles or silver ions being released?”
Just as silver as a metal has hardly any effect, the silver nanoparticles on your keyboard are “without effect”. The antibacterial effect occurs as soon as silver ions are released from the particles. In fact, however, the quantity of silver used in the keyboard coatings is so small that the amount of released ions is quasi irrelevant. Far more ions are released from e.g., sterling cutlery when in contact with food, water, acids or other substances. Moreover, you never known whether your keyboard’s silver coating is real or just a faked advertising gimmick.
About 4.7 million tons of pigmentary titanium dioxide (non-nano) are produced annually. The amount of nanoscale titanium dioxide produced is less than 1 percent thereof (less than approximately 47.000 tons).
After consulting an international manufacturer of chocolate bars, neither nanotechnologies at all nor nanoparticles were and are used in conjunction with their chocolate.
A patent contains a method for coating in the “plasma”, but this meas that the chocolate melts. The patent was never applied! Other foods can not be exposed to a plasma too.
Question: “Shopping for shoes, I came across some kind of a nanobased shoe care spray. The substances contained are distributed as aerosols whose potential hazards are discussed in numerous articles. Considering this and the present state of knowledge, is it justified to have such sprays approved?”
Since consumer safety has priority, the approval of such products is subject to legal regulations. In spite of this, however, legislation cannot always check and verify all substances, and dubious products may well appear on the market. Due to the very fine aerosols and containing solvents and active components compressed-gas sprays are often labeled as hazardous to health and should be used according to instructions.
Classification and approval have been required even before such “nanosprays” have been existing. Serious accidents already happened in the 70s of the 20th century with leather sprays or textile sprays, i.e. with surface-active substances applied to leather or to textiles. The fine mists of these products are easily inhaled by the consumer to settle down in parts of the lung tissue and impair health. This is also true for sprays containing the alleged “nanoparticles”, since sprays of that kind also depend on the use of additives and solvents. The pressurized dispensers are marked ”harmful to health“ and should be used outdoors, protected from the wind! To our knowledge, there are not any sprays available that really contain nanoparticles.
There are some websites that list producs with nanotechnology, for example:
- The Nanodatabase (Englisch): http://nanodb.dk/en/search-database/
- The Project of Emerging Nanotechnologies (in English): https://nanotechproject.tech/cpi/
For cosmetic products labelling of “nano” ingredients is mandatory since July 2013 and for food and food contact materials since Dec 2014.
Question: “Do particles pass over into the food? Will this cause health hazards?”
As a rule, cookware non-stick coatings have nothing to do with the nanoparticles discussed hereunder but consist of very heat-resistant polymers applied in layers. The effect of the surfaces obtained that way is similar to the well-known roll-off effect of the lotus plant.
Since manufacturers mostly make a mystery (patent) out of these kinds of coatings, there is no exact information about their possible hazards. However, since only „safe consumer products“ are allowed on the market, the non-stick coatings are assumed to be safe as well.
To what extent are nano sealings of glass, lacquer, or metal surfaces hazardous to the human organism – during the process of sealing or later, when the surface-treated products are actually used?
These are our opinions on the topic, we exclude any liability claims.
The term “nano” is not protected. No one guarantees that the corresponding product really contains nano. Some manufacturers use the term because it seems to be effective in advertising. The lacquer association has conducted a study that examines nanoparticles in lacquers (in German only). The result is described as being positive (from the customer’s point of view). https://www.wirsindfarbe.de/service-publikationen/sonstige-veroeffentlichungen/vdl-veroeffentlicht-broschuere-ueber-die-freisetzung-von-nano-objekten-aus-beschichtungen/
If no information about the used nanomaterial is provided on the product you can contact the manufacturer to get information about the used nanomaterials. I.e. which ones were used, where do they come from, are there any toxicologic studies on them, did the lacquer association conduct any tests on the product you are interested in? In the best case it can be concluded from these facts whether a risk does exist or not.
Topic: recycling, recovery, secondary raw material
There is no difference to existing products – if they are recyclable products they are recycled. Because nanoparticles often consist of rare materials (silver nanoparticles or rare earths) it will be in the interest of the companies to recycle these materials to prevent a steep increase in the prices of these products on medium-term. Therefore, there will be a self-regulation of the market.
Environment
Due to their small size (1 nm – 1 µm), nanoplastic particles can overcome certain barriers and accumulate in organisms or environmental compartments. In addition, undesirable chemicals such as flame retardants or plasticizers can bind to the nanoplastic particles and be released later, e.g. after uptake in environmental organisms. Currently, the estimated concentration of nanoplastics in the environment is very low and has no serious impact on plants and animals.
Microplastic (1 µm – 1 mm) particles currently pose a greater threat to humans and the environment due to higher measured environmental concentrations. Research groups around the world are currently working on this topic. It is expected that the number of microplastics as well as nanoplastic particles will strongly increase worldwide in the next decades via the gradual decomposition of plastics in the environment.
- For more information on this topic, please refer to the article in our cross cutting section “Nanoplastic in the environment”
In the wastewater treatment plant, contaminants including nanomaterials are separated from the wastewater in several stages. According to laboratory tests, the most common nanomaterials such as silicon dioxide, titanium dioxide, silver or zinc compounds are effectively removed from the wastewater by 90-95%. Only a small fraction of nanomaterials remains in the treated water. The majority of the removed nanomaterials are found in the sewage sludge, which is further treated separately.
- For more information on this topic, please refer to the article in our cross cutting section “Nanomaterials in the wastewater treatment plant”.
No, studies to date cannot prove that nanomaterials pose a risk to our wastewater treatment plants. But a possible cause of concern is the targeted use of nanomaterials with antibacterial properties. They could kill the bacteria in the biological treatment stages. However, the amounts of nanomaterials in the wastewater and later in the sewage treatment plant are too small to disrupt the work of the bacteria in the biological purification stage.
- For more information on this topic, please refer to the article in our cross cutting section “Nanomaterials in the wastewater treatment plant”
Currently, it is very difficult and tedious to directly detect engineered nanomaterials in the environment. There are still large knowledge gaps about the exposure, interactions and residence time of nanomaterials in the environment. Computer models can help to simulate such complex situation. However, this requires making certain assumptions and simplifications so that the theoretical values do not reflect the real quantities of nanomaterials in the environment. Such model calculations are a good tool for estimating risks and interactions of nanomaterials in the environment.
For more information on this topic, please refer to the article in our cross cutting section “Estimating the occurrence of nanomaterials in the environment”
Pollinating insects may encounter nanomaterials via the pollen, which is contaminated via application of nanomaterial-containing pesticides or fertilizers brought out on crops, and from traffic exhaust. While laboratory studies showed that nanomaterial can be harmful to some insects, it is unclear whether the low concentrations of nanomaterials found in outdoor environments do harm pollinators.
Read more about this topic in our cross cutting articles
Nanomaterials are used for the clean-up of soil and water highly contaminated with pollutants from various sources, mostly due to industrial activity. They are injected into the environment to effectively destroy the pollutants. The intentional release of nanomaterials warrants a careful safety assessment for environmental organisms prior to the actual application to avoid any negative effects on animals and plants. The benefits of using nanomaterials for environmental remediation should always outweigh the potential risks since the removal of highly toxic pollutants offers huge advantages for the environment.
Cleaning polluted water or soil from hazardous substances is time- and cost-intensive. Up-to-now the contaminated medium (water, soil) needs to be removed from the environment, cleaned, and then brought back into the original system. If a clean-up is not possible, the water or soil needs to be treated as waste, and is either burned or dumped in landfills. Here nanomaterials offer two advantages: (1) They can be directly introduced into the contaminated environment and (2) the removal of contaminants is more effective due to the higher reactivity of the nanomaterials.
Examples for these kind of applications have been intensively studied in the BMBF-funded projects Fe-NANOSIT, NanoSan and NAPASAN.
This is indeed a big challenge, because size and shape of natural and engineered nanoparticles may be quite similar, and mostly the natural particle outnumber the engineered ones. Hence, sophisticated analytical methods are applied, often by combining several methods, to unequivocally detect the engineered nanomaterials. For example, one uses impurities or different element or isotope ratios.
- Read more about this topic in our cross cutting article – “Detecting nanomaterials in the environment”!
Since only very small amounts of nanomaterials are found in the environment, the detection is very difficult and methodologically very demanding. The high natural background of particles complicates this even further. Moreover, since the analysis is very complex, time-consuming and expensive, synthetically engineered nanomaterials have only been detected in the environment in a few cases so far.
- Read more about this topic in our cross cutting article – “Detecting nanomaterials in the environment”!
No, currently there are no provisions that demand a specific disposal of nanomaterial-containing products. Liquid as well as solid waste containing nanomaterials should be disposed via the existing waste collection systems in order to prevent release into the environment. Currently there are many research activities dealing with nanomaterial fate during incineration and landfills.
- Read more about this topic in our cross cutting article – “Nanomaterials in waste”!
The nanomaterials will remain within the environment. What happens there in the long term cannot easily be answered with current methods. A major obstacle is the high natural background of the respective material in the environment. This makes it hard to distinguish between natural occurring and specifically introduced nanomaterials used for remediation purposes (e.g. iron). In any case, the nanomaterials will undergo transport and transformation processes (see basics article: transport & transformation, in preparation). However, most nanomaterials do not travel very far in the environment as they have a strong tendency to bind to other particles, e.g. soil particles.
To date there is no regulation in EU waste legislation to nanomaterials. The European Sewage Sludge Directive (86/278) aims to control the use of sewage sludge in agriculture so that harmful effects on soil, vegetation, animals and man are prevented, while a proper use of sewage sludge wil be promoted. These regulations and strict requirements on the basis of water law and chemicals law have meant that the pollutant content in municipal sewage sludge is decreased in recent years in some cases by more than 90 percent.
Source BMUV: https://www.bmuv.de/en/topics/water-resources-waste/circular-economy/types-of-waste-waste-flows/sewage-sludge
Question: “How dangerous are nanoscale particles (ultrafine particles) that are already present in the environment through soot emissions and natural aerosols like SiO2 or beech dust?
Dust and aerosols in the environment can also be hazardous. It is known that i.e. inhaled wood dust from beech and oak in joineries can lead to cancer. Fine dust and diesel exhaust gases are known to have an impact on the human respiration system.
Research on the effects of nanoparticles will lead to new findings regarding the evaluation of ultrafine particles occurring in the environment. Vice versa, present evaluation studies of fine dust and particles from combustion processes can lead to conclusions about the effects of some synthetic nanoparticles (i.e. in the respiratory tract).
Human body
Yes, but only very small amounts of nanomaterials can be taken up via the gastro-intestinal tract by penetrating tissue deeper layers and entering the blood stream. However, the overall amount is very low and most of the particles are not synthetic ones. Many minerals and other substances have a size range of about 1 to 100 nm, thus, our gastro-intestinal tract is very well adapted to handle such materials.
- Further information on this topic is provided in the body barriers article “nanoparticles and the gastro-intestinal tract”!
Yes, it has been shown that ultrafine dust particles including nanoparticles can cross the ultrathin tissue barrier in the lung thereby entering the body via the bloodstream. However, less than one-thousandth of the initially inhaled particles actually end up in the bloodstream so the amount is negligible.
- Further information on this topic is provided in the body barriers article “nanoparticles and the lung“!
Yes this is possible. But the mechanism and what physicochemical properties of the nanoparticles affect the transfer is still largely unknown and the subject of current research.
Please refer to the cross-cutting article “Nanoparticles at the placental barrier“.
Researchers are trying to shed light on open issues such as these. In spite of intensive efforts that serve to develop treatments for e.g. brain tumors which cannot be treated otherwise, it seems to be rather difficult so far to intentionally force sufficient quantities of nanoparticles over the blood-brain barrier. In view of this fact, it is rather improbable that nanoparticles other than the specially tailored ones will pass the barrier unintentionally.
For all that, it was found that nanoparticles can reach the brain by passing through the olfactory nerve. All experimental results obtained so far, however, indicate that the quantity of nanoparticles reaching the brain either via the blood-brain barrier or via the olfactory nerve in the olfactory epithelium of the nose is very small. Further research remains to be done to solve these issues.
- More information on nanoparticles and the brain can be found in the cross-cutting section at “nanoparticles at the blood-brain barrier” !!
Research
Safety and potential risks of nanomaterials is a big research topic both on a national and international level. Our projects’ section together with the project landscape offers a good overview on the involved German actors from the BMBF-funded nanosafety research projects (funding initiatives NanoCare4.0, NanoCare, NanoNature, ERA-Net SIINN). Further sources of information are e.g. the competency maps on nanotechnologies, the Environmental Research Database UFORDAT or the links section on the DaNa website. The links section lists relevant national and international networks dealing with nanotechnology topics, e.g. the European network NanoSafetyCluster.
In 2009, the total federal funding in Germany for R&D into nanotechnologies amounted to 382 million €, of which approximately 354 million € were contributed by the German Federal Ministry of Education and Research (BMBF).
Medicine
Nanomaterials are contained in many medical devices or applied to the surface. Examples and explanations can be found in our cross-cutting acticle on “Nanomaterials in medical devices“.
The use of nanomaterials in medical devices is clearly regulated at European level. Information on this can be found on the pages of the EUON Medical Devices at https://euon.echa.europa.eu/medical-devices.
In principle, that is true.
The antibacterial effect of silver nanoparticles is known. This is the reason why it is used as a coating for implants or in wound dressings. The effect is based on the release of silver ions, i.e. small, electrically charged particulate matter. Silver nanoparticles have especially good properties, as they have a large surface from which these ions can be released. Scientific studies show that besides the antibacterial effect these ions act as antiviral agents as well. Laboratory tests have shown that they are effective against certain types of corona virus family. Scientists are currently investigating whether this applies to the originator of the disease COVID-19 and are looking into the use of surface coatings with silver nanoparticles in hospitals and public places.
Further information can be found here:
- HeiQ AG – HeiQ Viroblock NPJ03 Antiviral textile Technology tested against effective corona virus (press release, 16.03.2020), https://www.heiq.com/news/heiq-viroblock-antiviral-textile-technology-against-coronavirus/
Yes, drugs available in pharmacies, shops, at the doctor’s or in hospitals may contain nanoparticulate ingredients because of their specific use to improve or enhance their efficacy. Both nanoparticles and liposomes are used for these purposes (see also “What is the difference between nanoparticles and liposomes?“). The number of drugs containing nanomaterials in the regulatory process is still low. These include, for example, drugs for the treatment of tumour diseases, chronic hepatitis, acromegaly (giant growth), multiple sclerosis, Crohn’s disease, age-related macular degeneration (AMD) with elevated LDL-C values or type 2 diabetes (see also or crosscutting text “Nanomedicine“).
In addition to the active ingredient, drugs also contain fillers and additives such as water, starch, vaseline or highly dispersed silicon dioxide. Due to the production process, silicon dioxide nanoparticles may also be generated. At present, pharmaceutical manufacturers are not obliged to label nanoscale ingredients in their medicines.
Further information can be found on the following websites of the European Medicines Agency (EMA)
Although liposomes are often referred to as nanoparticles, they differ from classical nanoparticles in both, their structure and in their stability . Liposomes are therefore not nanoparticles in a narrower sense.
Nanoparticles are made of solid materials. Liposomes can be between a few nanometers to even 10 microns in size. They consist of certain lipids (so-called phospholipids, e.g from soy) together with other materials and form a hollow sphere consisting of one or more double membranes (bilayers – see Fig. 1, liposome with a double membrane). They are filled with water and require a water-loving environment. Their bilayers are water-loving on the outside and also inside of the hollow sphere. They are usually less stable than nanoparticles.
The encapsulation system “nanosome” is very similar to the liposomes. Nanosomes, however, possess only a single lipid monolayer. The name refers to their extremely small size. The name Nanosome is mainly used in cosmetics.
Allergies are negative responses of the immune system towards substances that are tolerated by most people. This effect has never been observed for engineered nanomaterials. Reports about nanomaterials that are associated with a higher allergic risk are usually referring to fine dust. These ultrafine particles usually occur as components of environmental pollution, originating mainly from fire, agriculture and traffic.
Present research focuses on simultaneous exposure towards allergens and nanoparticles. Likewise, this principle is also being explored for the development of new therapies for allergies.
- Further information on this topic “nanoparticles and the immune system” can be found in our cross-cutting section !!!
Literature
Himly M., Grotz B., Sageder M., Geppert M., Duschl A. (2016). Immune Frailty and Nanomaterials: The Case of Allergies. Current Bionanotechnology, 2(1): 20-28.
There is still only a very small number of pharmaceuticals that according to the relevant admission data contain nanomaterials. Among them are drugs for:
- treatment of tumor diseases (e.g. Caelyx, Mepact, Abraxane, Rapamune, Renagel)
- chronic hepatitis (e.g. PegIntron, Pegasys)
- acromegaly (e.g. Somavert)
- multiple sclerosis (e.g. Copaxone)
- febrile neuropathy (e.g. Neulasta)
- Morbus Crohn (e.g. Cimzia)
- age-related macular degeneration (AMD) (e.g. Macugen)
- increased LDL-C values and diabetes mellitus type 2 (e.g. Welchol)
- MRT contrast agents (in-vivo diagnostics) with iron oxide nanoparticles (e.g. Feridex)
- parenteral iron (e.g. Cosmofer, Ferrlecit)
Besides, several authors refer to drugs containing nanoscale molecules and particles:
- liposomes (Caelyx, Myocet)
- polymer-protein conjugates (PegIntron, Somavert)
- polymeric substances (Copaxone)
Source : German Bundestag, Federal printed matter 17/3771.
Such vaccines against flu viruses don’t contain any synthetic nanoparticles as they wouldn’t have any task. Because the contact to the antibody-producing cells is made by injecting the vaccine directly into the blood, no “fillers” are needed.
The Paul-Ehrlich Institute (in Germany) gives the following information on its website:
…..”Although some of the components are in a size range of nanoparticles, it is not about synthetic nanoparticles.”
Legal
In the European Union, nanomaterials, which are purposely used as food additives, have to be labeled with the suffix “nano”. This does not apply to nanomaterials that are not specifically used in food, but which may arise as byproducts during the manufacturing process.
- For more information on this topic, please refer to the article in our cross cutting section “Nanomaterials in Food”
No, they do not. All chemicals including those used to enhance textile fibres are subject to the European chemicals legislation (REACH) or even stricter regulations such as the biocides’ regulation and have to be approved in this context. Textiles for normal use in everyday life have neither to be tested nor approved by anyone.
- Further information on this topic “nano in textiles” can be found in our cross-cutting section!!!
Yes, the EU Biocidal Products Regulation has specific provisions for nanomaterials. These provisions apply for active and non-active substances. If a nanoform of an already approved pesticide shall be used, the nanoproduct needs extra approval by submitting a dossier with all required data.
- Read more about this topic in our cross cutting article – “Nanomaterials in plant protection products”!
With nanotechnology being a cross-sectional technology, the nano-specific aspects are being addressed in various European directives and regulations. These include the legal areas of chemicals, food & food contact materials, pesticides and biocides, cosmetics, pharmaceuticals, medical devices, occupational health & safety and environmental protection.
The website of the German Federal Institute for Occupational Safety and Health (BAuA) provides information on regulations and preannouncements for workplace safety. The BAuA announcement “Manufactured Nanomaterials” (BekGS 527) from 2013 contains assessment values for the safe use of nanomaterials at the workplace. REACH, the European regulation on the Registration, Evaluation, Authorisation and Restriction of Chemicals, also includes the nanoform of substances without specifically addressing and assessing the nanoform yet. Since 2013 and 2014 respectively, regulations of cosmetics, biocides and food legislation include labelling requirements for nanosized ingredients. Further information on nano-specific regulations can be accessed via the following links
Germany
- Federal Institute for Occupational Safety and Health (BAuA) – Nanotechnology
- BAuA Announcement BekGS 527 “Manufactured Nanomaterials”
- REACH-CLP Helpdesk Germany
- German Social Accident Insurance (DGUV) – General Information on regulations for nanotechnology
Switzerland
- Federal Office of Public Health (FOPH) – Current Law
Austria
- NanoinformationPortal – Regelungen (in GERMAN)
- REACH-CLP Helpdesk Austria (in GERMAN)
For a better information of the consumers since Jul 2013 (cosmetics) and Dec 2014 (food) it is regulated by law (EU), that not only “titanium dioxide” is in the list of ingredients, but also the information is added, whether this is nanoscale [titanium dioxide (nano)]!
Also regulated by law is that only safe and approved ingredients may be included in cosmetics and foods, which have been sufficiently tested for possible negative effects. Therefore, this is by no means a threat notice, but only an information about the ingredients!
This depends on where they are to be used. For some product groups, the handling of nanomaterials is regulated very precisely:
Cosmetics
Dyes, preservatives and organic /inorganic UV-blockers may only be used in cosmetics if they have undergone a safety assessment and are explicitly approved for the respective application. This also applies if they are available as nanomaterials. Additional requirements then apply to the safety assessments. If nanomaterials are to be used for other purposes, they do not have to be specifically approved, but must be notified to the EU Commission. This notification also includes all information on size and coating for the respective application, properties, toxicological profile and safety data. In both cases, the Commission thus knows in which particle size, purity and composition as well as with which coatings or impurities the nanomaterials are used in cosmetics. Substances which have not been authorised or registered in this way are prohibited for all types of cosmetic products.
Food
Nano-ingredients for food must always be approved. If it is a completely new, previously unused ingredient that is considered a “technically produced nanomaterial” within the meaning of the law, this ingredient must be specially evaluated and approved. The same applies to food additives: whether nanomaterials or not – additives may only be used after prior testing and approval and only for precisely defined applications in food. This also applies to additives that have already been authorised. If such a substance is produced differently (e.g. nano-small) or used with a new function, it is considered to be a completely new substance and must undergo the approval procedure again from the outset. So far, there are no approved nano-ingredients on the European market.
So-called nanocapsules are often used in food supplements to keep vitamins and minerals dissolved or to transport them to the correct place in the body. However, these nanocapsules are not independent additives, but structures that form due to the chemical properties of their components. They have neither new properties nor their own biological effect and are therefore not considered nanomaterials by law. However, the “building blocks” from which they are formed are approved food additives.
Packaging
In plastics for materials intended to come into contact with food – packaging, storage containers, pots, refrigerator interiors, etc. – nanomaterials can only be used if they have been tested for safety and approved for the application in question. But even for food contact materials that are not made of plastic, manufacturers must in any case ensure that they do not release any substances into the food and pose a risk to health. They must therefore also keep an eye on nanomaterials and their behaviour. Antibacterial coatings made of nano-silver for foils and tableware, which are occasionally advertised on the Internet, are then not permitted in the EU.
Biocides
Products that are intended to be effective against microorganisms and pests must undergo an approval procedure in the EU. The approval then applies to the active substance and its specific use, and additional safety requirements may be imposed (labelling, warning, etc.). Nanomaterials need their own approval, even if the active substance has already been approved in its larger version. In addition, the biocidal products, as they are ultimately to be used, must also be tested and authorised as a whole. For this purpose, the effects of the product on humans, animals and the environment are investigated and evaluated. If a product contains nanomaterials, these effects must be determined specifically using methods that have been proven to do justice to the special properties of nanomaterials.
Other product groups
Drugs and medical devices undergo very complex and extensive safety tests. Although there are no nano-rules of their own, the existing approval procedures also cover them. There are no special approval procedures for textiles, materials, paints and all other product groups. Here too, however, manufacturers must ensure that their products are safe in the intended and foreseeable application.
In the EU, all chemicals must be registered, evaluated and authorised for use in Europe. This has also applied to nanomaterials since 2020. The registrant must carry out a risk assessment for humans and the environment for the respective registered forms. All actors within the supply chain, both registrants and downstream users, who are subject to the REACH regulation must collect and forward specific data for the nanoscale substances.
Many companies promote their products as “nano” because nanotechnology is in vogue today: Software companies, for instance, offer “nanotools”, i.e. small additional programs that adapt existing software to the special needs of computer users. Tata “Nano” is a very small car manufactured by the Indian vehicle manufacturer Tata Motors. Car washs praise their “nano” polishes which contain finest substances for extra-brilliant finishes.
For all that, nanomaterials are not always used or contained in the respective products. Only cosmetics, food and biocides are obliged to label nanomaterials. In the list of ingredients, “(nano)” then appears behind the name of the respective substance. The conditions under which manufacturers may voluntarily advertise with “nano”, however, are not regulated.
A “nano-notice” on the display side of packaging or in advertising may primarily be there to attract attention. Nevertheless, it can certainly be true. Industry and research have been doing experiments with very small structures for many years without necessarily having systematically developed them as “nanomaterials” in the sense of legal definitions. For example, nano-polishes for cars work by forming nano-fine surface structures after application. Micelles and liposomes, which encapsulate active ingredients in cosmetics and dietary supplements and keep them soluble, are also nano-small, but are produced solely by the chemical properties of their building blocks. And for many years titanium dioxide has been used as a finely ground inorganic (also called mineral) sunscreen, before it became subject to labelling.
In Germany and at European and international level there are no specific legal requirements on nanotechnology. Chemicals (this includes nanomaterials) are subject to the Chemicals Act, and the safety and health protection of employees at the workplace is subject to the Occupational Health and Safety Act.
In addition, since 1st July 2008 the European chemicals legislation REACH has provided a framework for the registration of nanomaterials. Research projects are being carried out to determine whether there is a need for specific action.
Since 2013, nanomaterials must be labelled in cosmetic products. From EU Regulation No. 1223/2009: “All components in the form of nanomaterials must be clearly listed in the list of components. The designation of such ingredients must be followed by the word “nano” in brackets.”
Since 13.12.2014, this also applies to the food industry: From EU Regulation No. 1169/2011: “All ingredients present in the form of technically produced nanomaterials must be clearly listed in the list of ingredients. The designation of such ingredients must be followed by the word “nano” in brackets.”
Material properties
Yes. Hazardous properties like flammability or explosiveness of certain substances or materials also apply to their nanoform. Here the material reacts with the oxygen from the air (oxidization) and releases energy in form of radiation / heat or as a shock wave (explosions). Especially oxidable metals and metal powders have this pyrophoric (spontaneous combustion) property.
The smaller the particle size of a flammable substance is, the easier it is to ignite the material thereby increasing its flammability. Nanomaterials have a lower ignition temperature compared to microscale particles and can be oxidized faster due to the higher specific surface area. The same is true for the explosiveness of flammable powdered nanomaterials. First the flammable nanomaterials and their respective agglomerates have to be finely distributed in the air (dust formation). Then, the air-nanomaterial-mixture is ignited resulting in an explosion. Usually, significantly less minimum energy is required to ignite nanoscale materials compared to their macroscale form.
Standardised testing methods like DIN EN 13501 are used to assess the flammability o different materials. In 2016, a new ISO standard was published for the testing of explosive materials (ISO/IEC 80079-20-2:2016).
Further information on this topics can be found online:
- German Federal Institute for Occupational Safety and Health (BAuA) – Fire and explosion hazards
- German Social Accident Insurance (DGUV) – Nanotechnology
- Swiss Federal Office of Public Health (FOPH) – Nanotechnology
Question: “Why, for example, are titanium dioxide nanoparticles transparent once they have reached a certain size?”
This is due to a physical effect. Any object that is clearly smaller than the wavelength of the visible light is invisible. Visible light is composed of wavelengths in the range of approximately 380 – 790 nanometers.
Particles that measure e.g. 100 nanometers are not visible anymore. This, however, happens only under extraordinary conditions: As soon as several particles are found one in front of or beside the other, they (normally) take on a white color and become visible again due to e.g. diffraction or dispersion. In spite of this, not all of the particles’ chemical and physical properties change at the nanolevel. Absorption properties, for example, persist i.e., the particles do not reflect light anymore, thus are transparent but actually absorb UV radiation.
Sustainability
A life cycle assessment (LCA; also called ecobalance) is a method for analysing environmental impacts of a product or service that considers the entire life cycle, starting with the procurement of the raw material, through processing, distribution, use and end-of-life disposal. A life cycle analysis calculates in detail for a product or service the energy and resource use and the potential health and environmental impacts. For this purpose, various indicators such as energy and raw material consumption or the release of pollutants are taken into account. Life cycle analysis can be used, for example, to determine which of two products uses fewer resources or which is more environmentally friendly. There are different types of life cycle analysis, which, among other things, consider different phases of the life cycle of a product. One aspect, for example, is the calculation of the carbon footprint.
The ISO 14044:2006 standard specifies certain requirements and provides guidelines for life cycle analysis.
In the linear economy, also called the “throwaway economy”, large parts of the raw materials used are landfilled or incinerated after the respective use phase of products. In contrast to that, the circular economy is designed to recycle a large part of the raw materials used after product usage, or to extend the use phase of a product through durable construction. Other measures include reducing emissions and energy use, maintenance, repair and reuse. Recycling is seen as the last means of choice in the circular economy.
In addition to measures such as reforestation or moorland rewetting, material innovations are an instrument against climate change. Innovative materials in products such as solar cells or batteries, for example, can help to ensure that more solar energy is converted into electricity, regenerative energies are better stored, fewer raw materials are used and overall fewer climate-damaging emissions are produced. Combined with improved recycling strategies, these measures help to slow down climate change. But this can only succeed in connection with further societal transformation processes (e.g. mobility, urban development).
A sustainable life style helps to protect the climate. The mere use of public transport or the bicycle instead of the car by individuals saves a large amount of CO2. In addition, many more people can be transported at the same time by bus or train. Replacing old appliances with modern ones with high energy efficiency classes provides enormous energy savings (e.g. light bulbs with LED lighting). Innovative materials make it possible to generate renewable energy, for example. Hydrogen produced in this way (green hydrogen) can contribute to modern mobility.
Recycling is the process of reprocessing discarded reusable materials into a new product. By collecting, separating and recycling reusable materials, raw materials are recovered and resources are conserved. The recovered raw materials can be used for the production of new products and waste is avoided. However, recycling is only truly sustainable when the raw materials can be recovered with as little energy input as possible, and the resulting material is of high purity. For the sustainability of a product, the reduction of raw material use in production and a longer service life have a higher significance.
There is still no generally accepted definition of the SSbD concept (Safe and substainable by Design). In general, the SSbD concept applies to chemicals, materials, products and processes and thus also to innovative materials. The concept aims to integrate safety, recyclability and functionality of chemicals and materials, with the overall objective of minimising environmental impacts throughout their life cycle and along the entire value chain. The SSbD concept is currently being further developed and specific criteria for SSbD of chemicals and materials defined.
In this way, the SSbD concept supports the vision of the EU Chemicals Strategy for Sustainability (CSS). The aim of this strategy is to produce and use chemicals in a way that maximises their benefits to society while avoiding harm to the planet and people.
Source: European Commission, Joint Research Centre, Caldeira, C., Farcal, R., Moretti, C., et al., Safe and sustainable by design chemicals and materials : review of safety and sustainability dimensions, aspects, methods, indicators, and tools, Publications Office of the European Union, 2022, https://data.europa.eu/doi/10.2760/879069
Planetary Boundaries describe a concept that defines nine environmental boundaries for our planet: Climate Change, New Substances and Modified Life Forms, Stratospheric Ozone Loss, Atmospheric Aerosol Content, Ocean Acidification, Biogeochemical Fluxes, Freshwater Use, Land Use Change and Biosphere Intactness.
Human-induced perturbations of Earth systems (e.g. increase in atmospheric CO2, ocean acidification) are calculated and visualised for each of the boundaries. As long as humans operate within the stress limits, humanity act within a “safe operation space”. Crossing one or more planetary boundaries carries the risk of abrupt environmental changes that can be harmful or even catastrophic.
Within the concept of planetary boundaries, economic systems and societies are embedded in the biosphere and therefore depend on its preservation. It sees the economy as an integral part of our society that must develop exclusively within planetary boundaries. Four planetary boundaries are defined as non-negotiable, namely: drinking water, climate, biodiversity and oceans. Therefore, according to the concept, sustainability goals 6 (water), 13 (climate), 14 (aquatic life) and 15 (terrestrial life) are of fundamental importance.
Education
Nanotechnology is used in a wide range of industrial sectors either manufacturing or applying nanoproducts. These include the sectors chemistry, electronics, mechanical engineering and pharmacology. For the company-based vocational training, relevant aspects of nanotechnology are incorporated within existing training professions, which may vary depending on the industry.
In Higher Education (college, university), there are a number of different nano-related courses for bachelor, master and doctoral degree programs. In addition to the nano-specific topics nanotechnology, nanoscience, nanomaterials or nano-biotechnology, this topic is also being addressed by other disciplines like chemistry, physics, materials sciences, biology and related application areas. In order to get a job in this field, a special course of studies on nanotechnology might be interesting but is not required in principle.
Further information on education possibilities for nanotechnology can be found on various online portals, e.g.
- Germany: www.studienwahl.de
- Austria: www.studienwahl.at
- Switzerland: www.berufsberatung.ch
Public debate
The German Federal Ministries of Education and Research (BMBF), Environment – Nature – Conservation and Nuclear Safety (BMUB), Labour and Social Affairs (BMAS), and Food – Agriculture and Consumer Protection (BMEL) have been spending approximately 14.18 million € per year on risk research projects and supporting research.
The EU sees nanotechnology as one of the leading technologies. See also the Lund Declaration from July 2009 “The Lund Declaration: EUROPE MUST FOCUS ON THE GRAND CHALLENGES OF OUR TIME (PDF-Document, 105 KB ).
The chemical industry is contributing to the social debate on nanotechnology in two ways: by providing information and by engaging in dialogues. Employees present their own research results to the public at conferences and in publications. Some companies inform about topics like work protection or about a nanotechnology code of conduct (EU-Codex ) on their websites.
The chemical industry is proactive on possible concerns and worries of people regarding their products. It participated with own works in research projects like NanoCare, INOS and TRACER and is involved in the public dialogue. The industry also takes part in other ongoing research projects like Carbosafe.
Question: “Is it reasonable to call for a regulation that prohibits further research on nanotechnology?”
Not from the point of view of DaNa and the NanoCare Cluster. There are indeed still some knowledge gaps, but that is this way with every new area of research. These gaps will be closed by the new findings from the numerous national, European and international projects. In our opinion there are so many positive aspects to nanotechnology (i.e. in the medical sector or in the protection of the environment.) that a moratorium would be contra productive.
As many processes in nature take place on the nanoscale, research on nanotechnology will lead to a better understanding of these natural processes. Advances in medicine would be very difficult if research on nanotechnology was not allowed any more. Of course the intended goals of every single project have to be validated. Ethically dubious projects are rejected by most scientists and all sponsors.
For the nanotechnologists their science is just like any other: what matters are the people working in this field and what they make of it.
Other
As nanoparticles are omnipresent in the natural environment, one cannot really protect oneself from them in daily life and dust masks help only partially. Up to 10,000 naturally occurring nanoparticles per cubic centimeter fly around in clean air. The smoke of one cigarette increases the amount of these particles to over 100,000 in the surrounding area. The natural nanoparticles derive from dust storms (e.g. in the Sahara), forest fires, volcanic eruptions, etc., and can be transported over large distances. Normally, this is nothing to worry about, because our body can handle these nanoparticles. Yet, in principle, protection is possible: In the nanoparticle industry, employers must provide appropriate protective equipment (fume hoods, clothing, masks with “nano-filters”) to ensure that employees are not endangered. For best possible protection, the maximum allowable values are reviewed annually.
No. It is electromagnetic.
 >
>